Key Points
MDS patients undergoing transplantation with haploidentical relative and matched unrelated donors had similar OS rates.
Relapse was more common in patients with haploidentical donors; chronic GVHD occurred more often in those with matched unrelated donors.
Abstract
We compared outcomes in 603 patients with myelodysplastic syndrome (MDS) after HLA-haploidentical relative (n = 176) and HLA-matched unrelated (n = 427) donor hematopoietic cell transplantation (HCT) from 2012 to 2017, using the Center for International Blood and Marrow Transplant Research database. All transplantations used reduced-intensity conditioning regimens. Total-body irradiation plus cyclophosphamide and fludarabine was the predominant regimen for HLA-haploidentical relative donor HCT, and graft-versus-host disease (GVHD) prophylaxis was uniformly posttransplantation cyclophosphamide, calcineurin inhibitor, and mycophenolate. Fludarabine with busulfan or melphalan was the predominant regimen for HLA-matched unrelated donor HCT, and GVHD prophylaxis was calcineurin inhibitor with mycophenolate or methotrexate. Results of multivariate analysis revealed higher relapse (hazard ratio [HR], 1.56; P = .0055; 2-year relapse rate, 48% vs 33%) and lower disease-free survival (DFS) rates after HLA-haploidentical relative donor HCT (HR, 1.29; P = .042; 2-year DFS, 29% vs 36%). However, overall survival (OS) rates did not differ between donor type (HR, 0.94; P = .65; 2-year OS, 46% for HLA-haploidentical and 44% for HLA-matched unrelated donor HCT) because of mortality associated with chronic GVHD. Acute grade 2 to 4 GVHD (HR, 0.44; P < .0001) and chronic GVHD (HR, 0.36; P < .0001) were lower after HLA-haploidentical relative donor HCT. By 2 years, probability of death resulting from chronic GVHD was lower after HLA-haploidentical relative compared with HLA-matched unrelated donor HCT (6% vs 21%), negating any potential survival advantage from better relapse control. Both donor types extend access to transplantation for patients with MDS; strategies for better relapse control are desirable for HLA-haploidentical relative donor HCT, and effective GVHD prophylaxis regimens are needed for unrelated donor HCT.
Visual Abstract
Introduction
Allogeneic hematopoietic cell transplantation (HCT) has been a successful strategy to treat myelodysplastic syndrome (MDS). Although prospective trials comparing HCT with non-HCT approaches for MDS are lacking, outcomes after HCT are promising. In a study that compared survival of patients with MDS who were candidates for HCT, based on donor availability, the 4-year overall survival (OS) rate was 37% for patients who underwent HLA-matched sibling donor HCT (n = 112) compared with 15% for patients who did not receive HCT because of lack of donor (n = 50).1 The observed survival advantage after HLA-matched sibling donor HCT was evident beyond the second year after HCT. Large registry studies of reduced-intensity conditioning (RIC) HCT for older patients with MDS have reported long-term survival rates ranging from 30% to 45%.2,3 Although the best donor for allogeneic HCT is generally considered to be an HLA-matched sibling, only ∼30% of patients have a suitable sibling.4 HLA-matched unrelated donor HCT has been used for decades for MDS, with reports documenting comparable long-term survival after HLA-matched sibling and HLA-matched unrelated donor HCT but lower survival rates after 1-locus HLA-mismatched unrelated donor HCT.5 Although most White patients are able to identify a suitable unrelated donor, this is not the case for non-Whites, and transplantation of grafts from HLA-mismatched unrelated donors is associated with higher mortality.4,5 Recent years have witnessed increasing use of HLA-haploidentical relatives as donors using posttransplantation cyclophosphamide (PTCY) for graft-versus-host disease (GVHD) prophylaxis for hematologic malignancies, including MDS.6-10 In these reports,6-9 acute myeloid leukemia (AML) was the predominant malignancy, and these studies are limited by their modest sample sizes. A recent study evaluated 228 MDS patients undergoing HLA-haploidentical donor HCT that included both myeloablative conditioning (MAC) and RIC regimens and GVHD prophylaxis using PTCY and other approaches.10 This report concluded that approximately one-third of patients were alive and disease free 3 years after HLA-haploidentical relative donor HCT. Collectively, existing reports confirm HLA-haploidentical relatives extend the donor pool for MDS and other myeloid malignancies.6-10 Because most patients are expected to have an HLA-haploidentical relative, the primary objective of the current analysis was to compare outcomes after commonly used strategies for HLA-haploidentical and HLA-matched unrelated donor HCT for MDS.
Methods
Patients
Data were obtained from the Center for International Blood and Marrow Transplant Research, a working group of transplantation centers that submit data on standardized reporting forms; patients are followed longitudinally. Forty-nine centers contributed patients to the HLA-haploidentical and 70 centers to the HLA-matched unrelated donor HCT groups. Patients underwent transplantation in the United States from 2012 to 2017. Patients age 50 to 79 years with de novo or therapy-related MDS were included. HLA-haploidentical relative donor transplantations were mismatched at ≥2 HLA loci, and unrelated donor transplantations were matched at HLA-A, -B, -C, and -DRB1 at the allele level. All patients received RIC regimens as previously defined,11 and all patients received T cell–replete bone marrow or peripheral blood. Excluded were patients with an MDS that transformed to AML, patients who had undergone prior allogeneic HCT, and those receiving in vivo T-cell depletion or ex vivo graft manipulation. Patients provided written informed consent. The Institutional Review Board of the National Marrow Donor Program approved the study.
Outcomes
OS was the primary end point. Other end points included hematopoietic recovery, acute and chronic GVHD, relapse, nonrelapse mortality (NRM), and disease-free survival (DFS). Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive days, and platelet recovery was defined as achieving a platelet count ≥20 × 109/L without transfusion for 7 consecutive days. Graft failure was defined as failure to achieve ANC ≥0.5 × 109/L, decline in ANC to <0.5 × 109/L without recovery after having achieved ANC ≥0.5 × 109/L, myeloid donor chimerism <5%, or second transplantation or donor leukocyte infusion.12 Disease relapse, progression, and death were treated as events. NRM was defined as time to death without relapse or progression. Relapse was defined as molecular, cytogenetic, or morphologic recurrence of MDS. DFS was defined as survival without relapse or progression. Grade 2 to 4 acute and chronic GVHD were graded using previously described criteria.13,14
Statistical analysis
The incidences of neutrophil and platelet recovery and graft failure were calculated using the cumulative incidence estimator.15 Propensity scores were generated for each patient using logistic regression based on age, Karnofsky performance score (KPS), use of hypomethylating agents (HMAs) pretransplantation, and Revised International Prognostic Scoring System (IPSS-R) score at transplantation. The median propensity score for HLA-haploidentical relative donor HCT was 0.41 (range, 0.01-0.92); for HLA-matched unrelated donor HCT, it was 0.22 (range, 0.01-0.83). Multivariate analyses were performed using Cox proportional hazards models for acute and chronic GVHD, relapse, NRM, relapse, DFS, and OS to examine the effect of donor type with adjustment for propensity score.16 A stepwise model-building approach was adopted, testing the following variables: sex, HCT comorbidity index (HCT-CI), recipient cytomegalovirus (CMV) serostatus, MDS type (de novo or therapy related), World Health Organization classification, interval between diagnosis and transplantation, and transplantation period (Table 1). Variables that attained a P value ≤.05 were retained in the final model. The final model included the variable for donor type regardless of level of significance and was adjusted for propensity score. The incidences of acute and chronic GVHD and the probabilities of DFS and OS, relapse, and NRM were calculated from the final Cox model.17,18 Transplantation center effect on survival was tested using the frailty model.19 All P values are 2 sided, and analyses were performed using SAS (version 9.4; Cary, NC).
Table 1.
Patient, disease, and transplantation characteristics
| Characteristic | n (%) | P | |
|---|---|---|---|
| HLA-haploidentical donor (N = 176) | HLA-matched unrelated donor (N = 427) | ||
| Age, y | <.001 | ||
| 50-59 | 38 (22) | 41 (10) | |
| 60-69 | 103 (59) | 250 (59) | |
| 70-79 | 35 (20) | 136 (32) | |
| Sex | .24 | ||
| Male | 123 (70) | 277 (65) | |
| Female | 53 (30) | 150 (35) | |
| Race | <.001 | ||
| White | 141 (80) | 405 (95) | |
| Non-White | 32 (18) | 14 (3) | |
| Not reported | 3 (2) | 8 (2) | |
| HCT-CI | .30 | ||
| 0-2 | 76 (43) | 165 (39) | |
| 3+ | 100 (57) | 262 (61) | |
| KPS | .004 | ||
| 90-100 | 87 (49) | 177 (42) | |
| <90 | 82 (47) | 246 (57) | |
| Not reported | 7 (4) | 4 (< 1) | |
| CMV serostatus | .22 | ||
| Negative | 58 (33) | 173 (41) | |
| Positive | 117 (66) | 252 (59) | |
| Not reported | 1 (<1) | 2 (<1) | |
| Disease type | .44 | ||
| De novo MDS | 152 (86) | 358 (84) | |
| Therapy-related MDS | 24 (14) | 69 (16) | |
| WHO classification | .02 | ||
| RA/RARS/RCMD | 39 (22) | 134 (31) | |
| RAEB-1/RAEB-2 | 97 (55) | 229 (54) | |
| MDS NOS | 40 (23) | 64 (15) | |
| Systemic therapy before transplantation | <.001 | ||
| HMAs | 139 (79) | 323 (75) | |
| No HMAs | 15 (9) | 87 (21) | |
| Not reported | 22 (12) | 17 (4) | |
| Cytogenetic risk | <.001 | ||
| Monosomal karyotype | 33 (19) | 63 (15) | |
| Good | 83 (47) | 224 (52) | |
| Intermediate | 34 (19) | 80 (19) | |
| Poor/very poor | 20 (11) | 54 (12) | |
| Not reported | 6 (3) | 6 (1) | |
| Bone marrow blasts before transplantation, % | <.001 | ||
| <2 | 88 (50) | 212 (50) | |
| 2-5 | 35 (20) | 84 (20) | |
| 5-10 | 26 (15) | 97 (23) | |
| >10 | 9 (5) | 25 (6) | |
| Not reported | 18 (10) | 9 (2) | |
| IPSS-R score before transplantation | <.001 | ||
| Very low | 3 (2) | 35 (8) | |
| Low | 25 (14) | 123 (29) | |
| Intermediate | 44 (25) | 138 (32) | |
| High | 43 (24) | 93 (22) | |
| Very high | 43 (24) | 30 (7) | |
| Not reported | 18 (10) | 8 (2) | |
| Disease risk index | .08 | ||
| Intermediate | 59 (34) | 128 (30) | |
| High | 114 (65) | 298 (70) | |
| Not reported | 3 (1) | 1 (<1) | |
| Interval from diagnosis to transplantation, mo | <.04 | ||
| <6 | 37 (21) | 132 (31) | |
| 6-12 | 67 (38) | 131 (31) | |
| >12 | 72 (41) | 164 (38) | |
| Conditioning regimen* | NA | ||
| TBI + cyclophosphamide + fludarabine | 143 (81) | 6 (1) | |
| TBI + fludarabine | 11 (6) | 73 (17) | |
| Busulfan + fludarabine | 5 (3) | 187 (44) | |
| Fludarabine + melphalan | 10 (6) | 147 (35) | |
| TBI + busulfan + fludarabine | 7 (4) | 14 (3) | |
| Transplantation period | <.001 | ||
| 2012-2014 | 41 (23) | 179 (42) | |
| 2015-2017 | 135 (77) | 248 (58) | |
NA, not applicable (confounded with donor type); NOS, not otherwise specified; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; WHO, World Health Organization.
TBI dose ≤500 cGy.
Results
Patient, disease, and transplantation characteristics
Patient, disease, and transplantation characteristics are listed in Table 1. In both treatment groups, most transplantations were performed for de novo MDS. In addition, most patients received HMAs before transplantation and had HCT-CI scores of ≥3. World Health Organization classification and cytogenetic risk differed by treatment group. Recipients of HLA-haploidentical donor transplantations were more likely to have very high IPSS-R scores at transplantation. There were other differences between the treatment groups. Recipients of HLA-haploidentical donor transplantations were more likely to be younger and non-White, have KPS of 90 or 100, receive bone marrow (although peripheral blood was the predominant graft for both donor types), undergo low-intensity total-body irradiation (TBI)–containing regimens (TBI at 200 cGy, cyclophosphamide, and fludarabine), and have a longer interval between diagnosis and transplantation. The median age of recipients of HLA-haploidentical donor transplantations was 65 years (range, 50-78 years); for recipients of HLA-matched unrelated donor transplantations, it was 68 years (range, 51-79 years). Recipients of HLA-haploidentical donor transplantations received uniform GVHD prophylaxis consisting of PTCY with calcineurin inhibitor and mycophenolate. Most recipients of HLA-matched unrelated donor transplantations received an alkylating agent (busulfan or melphalan) with fludarabine as their transplantation conditioning regimen, and all received GVHD prophylaxis that included calcineurin inhibitor with methotrexate (n = 296; 69%) or mycophenolate (n = 131; 31%). Neither treatment group received antithymocyte globulin or alemtuzumab. Peripheral blood was the predominant graft for both donor types. Sixty-two percent (110 of 176) of HLA-haploidentical relative donor transplantations were peripheral blood grafts, and the remaining 38% (66 of 176) were bone marrow grafts. Peripheral blood grafts accounted for 92% of HLA-matched unrelated donor transplantations (391 of 427). Only 36 (8%) HLA-matched unrelated donor transplantations were performed using bone marrow grafts. The median follow-up of survivors was 24 and 36 months for those undergoing HLA-haploidentical and HLA-matched unrelated donor transplantations, respectively.
Hematopoietic recovery
Hematopoietic recovery was slower after HLA-haploidentical compared with HLA-matched unrelated donor transplantation. The day-28 incidence of neutrophil recovery after HLA-haploidentical donor HCT was 88% (95% confidence interval [CI], 83%-93%) compared with 97% (95% CI, 95%-98%) for HLA-matched unrelated donor HCT (P < .0001). The corresponding day-100 incidences of platelet recovery were 83% (95% CI, 77%-89%) and 95% (95% CI, 93%-97%; P < .0001). The 1-year incidence of graft failure was higher after HLA-haploidentical compared with HLA-matched unrelated donor transplantation; graft failure rates were 15% (95% CI, 8%-24%) and 8% (95% CI, 5%-12%; P = .020), respectively. Thirteen recipients of HLA-haploidentical donor transplantations (bone marrow, n = 5; peripheral blood, n = 8) had graft failure, and 10 of these patients died. Six patients received a second transplantation after graft failure, and 1 patient underwent donor leukocyte infusion; only 3 of these patients were alive at the time of this analysis. Twenty-five recipients of HLA-matched unrelated donor transplantations (bone marrow, n = 2; peripheral blood, n = 23) had graft failure, and 23 of these patients died. Two patients received a second transplantation and were alive at the time of this analysis.
In a subset analysis that examined for an effect of graft type for recipients of HLA-haploidentical donor transplantations, the day-28 incidence of neutrophil recovery did not differ after transplantation of bone marrow (85%; 95% CI, 75%-93%) vs peripheral blood (91%; 95% CI, 84%-95%; P = .11). The day-100 incidence of platelet recovery was lower after transplantation of bone marrow (78%; 95% CI, 67%-88%) compared with peripheral blood (86%; 95% CI, 79%-92%; P = .04). When the analysis was limited to recipients of peripheral blood, consistent with the main analysis, neutrophil (97%; 95% CI, 96%-99%) and platelet (96%; 95% CI, 94%-98%) recovery rates were higher after HLA-matched unrelated donor HCT compared with HLA-haploidentical donor HCT (P < .001).
GVHD
The risk of grade 2 to 4 and 3 to 4 acute GVHD was lower after haploidentical relative compared with HLA-matched unrelated donor HCT (Table 2). The day-100 incidence of grade 2 to 4 acute GVHD was 24% (95% CI, 18%-31%) after HLA-haploidentical and 44% (95% CI, 40%-49%) after HLA-matched unrelated donor HCT. No other factors were associated with grade 2 to 4 or 3 to 4 acute GVHD. Similarly, the risk of chronic GVHD was lower after HLA-haploidentical relative compared with HLA-matched unrelated donor HCT (Table 2). No other factors were associated with chronic GVHD. The 2-year incidences of chronic GVHD were 22% (95% CI, 16%-29%) and 56% (95% CI, 51%-61%) after HLA-haploidentical relative and HLA-matched unrelated donor HCT, respectively. Among recipients of HLA-haploidentical donor HCT, the day-100 incidences of grade 2 to 4 acute GVHD were 27% (95% CI, 17%-38%) and 23% (95% CI, 16%-32%) after transplantation of bone marrow and peripheral blood, respectively (P = .64). The corresponding 2-year incidences of chronic GVHD were 15% (95% CI, 7%-25%) and 25% (95% CI, 17%-34%; P = .19). When the analysis was limited to recipients of peripheral blood, consistent with the main analysis, grade 2 to 4 acute GVHD (44%; 95% CI, 39%-49%) and chronic GVHD (58%; 95% CI, 52%-63%) were observed more frequently after HLA-matched unrelated compared with HLA-haploidentical donor HCT (P < .001).
Table 2.
Results of multivariate analysis adjusted for propensity score
| Outcome | HR (95% CI) | P |
|---|---|---|
| Grade 2-4 acute GVHD | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 0.44 (0.31-0.62) | <.0001 |
| Grade 3-4 acute GVHD | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 0.26 (0.14-0.48) | <.0001 |
| Chronic GVHD | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 0.36 (0.25-0.53) | <.0001 |
| NRM* | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 0.90 (0.62-1.31) | .57 |
| Relapse† | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 1.56 (1.14-2.14) | .0055 |
| DFS‡ | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 1.29 (1.01-1.65) | .042 |
| OS§ | ||
| HLA-matched unrelated | 1.00 | |
| HLA-haploidentical relative | 0.94 (0.71-1.24) | .65 |
Each patient was assigned a propensity score based on age, KPS, HMAs before transplantation and IPSS-R score at transplantation.
HR, hazard ratio.
Adjusted for recipient CMV serostatus.
Adjusted for recipient sex.
Adjusted for donor-recipient sex match.
Adjusted for HCT-CI score.
NRM and relapse
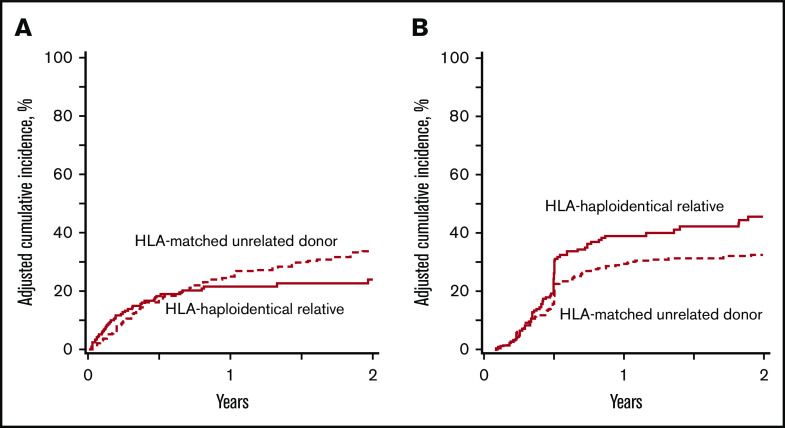
The 2-year incidences of NRM were 24% (95% CI, 18%-31%) and 34% (95% CI, 30%-39%) after HLA-haploidentical relative and HLA-matched unrelated donor HCT, respectively (Figure 1A). The corresponding incidences of relapse were 48% (95% CI, 39%-56%) and 33% (95% CI, 28%-37%), respectively (Figure 1B). The results of multivariate analysis are summarized in Table 2. Overall, NRM did not differ by treatment group after adjusting for recipient CMV serostatus. NRM was higher for CMV-seropositive recipients (hazard ratio [HR], 1.56; 95% CI, 1.12-2.16; P = .008); CMV seropositivity was the only characteristic associated with NRM after adjusting for propensity score. Relapse risk was higher after HLA-haploidentical relative compared with HLA-matched unrelated donor HCT (HR, 1.56; P = .0055; Table 2). The relapse rate was also higher in women than men (HR, 1.48; 95% CI, 1.11-1.97; P = .007). Interval from diagnosis to transplantation was not associated with relapse (HR, 0.97; 95% CI, 0.69-1.37; P = .88). Chronic GVHD had a significant effect on lowering relapse risk (HR, 0.53; 95% CI, 0.35-0.81; P = .003), but the effect of chronic GVHD on relapse did not differ by treatment group (P = .60). Grade 2 to 4 acute GVHD did not affect relapse risk (HR, 1.12; 95% CI, 0.83-1.50; P = .45).
Figure 1.
NRM and relapse incidence rates. (A) The 2-year incidences of NRM were 24% (95% CI, 18%-31%) and 34% (95% CI, 30%-39%) after HLA-haploidentical relative and HLA-matched unrelated donor transplantations, respectively. (B) The 2-year incidences of relapse were 48% (95% CI, 39%-56%) and 33% (95% CI, 28%-37%) after HLA-haploidentical relative and HLA-matched unrelated donor transplantations, respectively.
DFS and OS
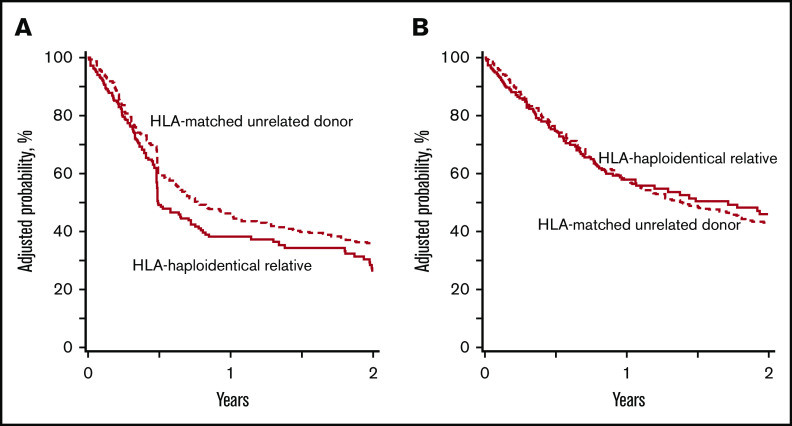
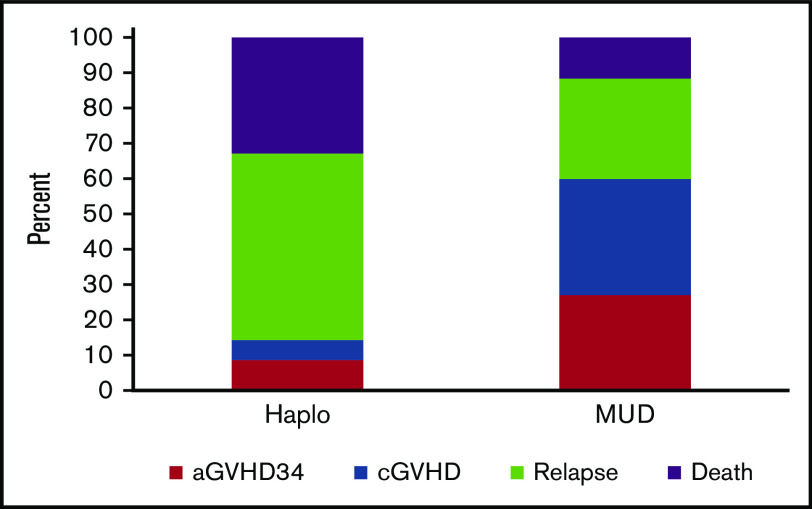
The 2-year probabilities of DFS after HLA-haploidentical and HLA-matched unrelated donor transplantation were 29% (95% CI, 21%-37%) and 36% (95% CI, 31%-41%), respectively (Figure 2A). The corresponding probabilities of OS were 46% (95% CI, 37%-54%) and 44% (95% CI, 39%-48%; Figure 2B). The results of multivariate analysis are summarized in Table 2. DFS was lower after HLA-haploidentical relative compared with HLA-matched unrelated donor HCT (HR, 1.29; P = .042). OS did not differ by donor type (HR, 0.94; P = .65). Survival was lower with HCT-CI score of ≥3 (HR, 1.30; 95% CI, 1.03-1.65; P = .026). Interval from diagnosis to HCT was not associated with DFS (HR, 0.95; 95% CI, 0.74-1.23; P = .71) or OS (HR, 1.00; 95% CI, 0.76-1.33; P = .98). The 2-year probabilities of GVHD-free, relapse-free survival were 23% (95% CI, 16%-30%) and 16% (95% CI, 13%-20%) after HLA-haploidentical relative and HLA-matched unrelated donor HCT, respectively (P = .14). The relative contributions of GVHD, relapse, and death to outcomes by donor type are shown in Figure 3. We examined for an effect of disease risk index (DRI) on OS and did not observe any difference by donor type (test for interaction P = .09). The 2-year probabilities of survival for intermediate DRI were 64% (95% CI, 50%-77%) and 57% (95% CI, 48%-65%) after HLA-haploidentical compared with HLA-matched unrelated donor HCT (P = .78). The corresponding 2-year survival rates for patients with high DRI were 34% (95% CI, 25%-45%) and 39% (95% CI, 33%-45%; P = .35). Recurrent MDS was the predominant cause of death in both treatment groups, but the proportion of patients whose death was attributed to recurrent MDS was higher after HLA-haploidentical compared with HLA-matched unrelated donor HCT (44% vs 33%; P < .001). The proportion of deaths attributed to GVHD was lower after HLA-haploidentical compared with HLA-matched unrelated donor HCT (7% vs 26%; P < .001). Further exploration confirmed that chronic GVHD was associated with higher likelihood of death. The 2-year probability of death resulting from chronic GVHD in recipients of unrelated donor HCT was 21% compared with 6% in recipients of HLA-haploidentical donor HCT. The day-100 probability of death resulting from acute GVHD in recipients of unrelated donor HCT was 17% compared with 12% in recipients of HLA-haploidentical donor HCT. Other causes of death did not differ by treatment group.
Figure 2.
DFS and OS probabilities. (A) The 2-year probabilities of DFS were 29% (95% CI, 21%-37%) and 36% (95% CI, 31%-41%) after HLA-haploidentical relative and HLA-matched unrelated donor transplantations, respectively. (B) The 2-year probabilities of OS were 46% (95% CI, 37%-54%) and 44% (95% CI, 39%-48%) after HLA-haploidentical relative and HLA-matched unrelated donor transplantations, respectively.
Figure 3.
GVHD and relapse-free survival. The relative contribution of grade 3 to 4 acute GVHD (aGVHD34), chronic GVHD (cGVHD), relapse, and death to outcomes by donor type. Haplo, haploidentical donor; MUD, matched unrelated donor.
Discussion
Increasing use of allogeneic HCT for older patients and for those with donors other than HLA-matched siblings have resulted in increasing numbers of transplantations for hematologic malignancies.20 Furthermore, in recent years, there has been an increase in use of HLA-haploidentical relatives as donors for MDS, a group of diseases for which the median age at diagnosis is ∼70 years.21,22 Therefore, the primary objectives of the current analyses were: 1) to report outcomes after HLA-haploidentical relative donor HCT with PTCY for MDS and 2) to compare these outcomes with outcomes after HLA-matched unrelated donor HCT during the same period. We observed a higher relapse risk after HLA-haploidentical relative compared with HLA-matched unrelated donor HCT that resulted in lower DFS after HLA-haploidentical relative donor HCT. The absence of a difference in OS between donor types is explained by higher chronic GVHD (with an adverse effect on survival) after HLA-matched unrelated donor HCT. Death attributed to chronic GVHD was 3.5 times higher after HLA-matched unrelated compared with HLA-haploidentical relative donor HCT. This negated any survival advantage that may have resulted from fewer relapses after HLA-matched unrelated donor HCT. It is plausible that a survival advantage attributed to lower relapse risk would require longer follow-up. In the setting of HLA-haploidentical donor HCT, acute and chronic GVHD rates did not differ by graft type. We hypothesize that the incorporation of PTCY into GVHD prophylaxis mitigated the GVHD risk. Choosing a suitable donor for HCT is complex and requires consideration of many factors, including donor availability, timing and cost of graft acquisition, and transplantation center expertise. With regard to MDS patients undergoing RIC HCT, the present analysis does not show a survival advantage for HLA-haploidentical relative or HLA-matched unrelated donor HCT. When considering an RIC regimen for HCT in MDS, HLA-haploidentical relative and HLA-matched unrelated donors both extend access to HCT. The outcomes after HLA-haploidentical relative donor HCT are encouraging for non-Whites, who are less likely to identify an HLA-matched unrelated donor.4 Although in the present analysis adjusted NRM did not differ by treatment group, NRM was higher for CMV-seropositive recipients. It is noteworthy that for non-White patients, finding unrelated CMV-matched donors may present a particular challenge.
Relapse risk after HLA-haploidentical relative donor HCT was higher than after HLA-matched unrelated donor HCT. Although all transplantations in the current analysis used RIC, the intensity of the regimen varied by donor type. Recipients of HLA-haploidentical donor transplantations primarily received what is regarded as a low-intensity regimen that included TBI at 200 cGy, fludarabine, and cyclophosphamide. In contrast, most recipients of HLA-matched unrelated donor transplantations received fludarabine with busulfan or melphalan, regimens considered higher intensity than the low-dose TBI regimen. A more appropriate comparison of donor sources would have been limited to patients who received fludarabine with busulfan or melphalan. Such a comparison could not be undertaken in the current analysis, because only 9% of HLA-haploidentical relative donor transplantations in this study used these regimens. In addition, studies aimed at comparing donor types that use PTCY-containing GVHD prophylaxis regimens for both donor types may further inform donor choices in the future.
A report that studied the effect of regimen intensity for HLA-haploidentical relative donor HCT for myeloid malignancies failed to show a difference in relapse risk between the low-dose TBI regimen and the more intensive regimen consisting of busulfan or melphalan with fludarabine.23 However, relapse risks are higher with RIC compared with MAC regimens.24,25 An effect of graft type cannot be examined in the current analysis, because graft type is correlated with donor type. In another study of HLA-haploidentical relative donor HCT for hematologic malignancies, relapse was higher after transplantation of bone marrow compared with peripheral blood but without a difference in DFS or OS.26
There are 2 recent reports on HLA-haploidentical donor HCT for MDS.10,27 The outcomes reported in the current analysis are inferior to those reported by Wang et al.27 However, the study populations differ markedly insofar as the current analysis includes a substantially older population. Additionally, all HLA-haploidentical donor transplantations in the current analysis were T cell replete and performed with PTCY for GVHD prophylaxis. Our findings are more in keeping with the report by Robin et al,10 in which 102 of 228 patients received PTCY for GVHD prophylaxis. In their analysis, the use of MAC regimens and use of PTCY for GVHD prophylaxis were associated with better outcomes.10 However, NRM was higher in the Robin et al study and may be attributed to a higher-risk population that included MDS transformed to AML and use of MAC regimens.10
The current analysis comparing 2 donor types has several limitations. There were several patient and disease characteristics that differed between the treatment groups. We assigned a propensity score for each patient based on age, KPS, hypomethylating agent use before transplantation, and IPSS-R score to accommodate for more homogeneous treatment cohorts. Additionally, the conditioning regimen and GVHD prophylaxis were packages associated with the type of donor chosen for transplantation, and as such, it was impossible to dissociate the donor type and the transplantation package. Consequently, any interpretation must consider the donor type as well as the regimen(s) offered for that donor type. Because haploidentical transplantations were more common after 2014, the median follow-up was 24 months compared with 36 months for HLA-matched unrelated donor HCT. Therefore, outcomes were censored at 2 years. Longer follow-up will allow for studying the effect of relapse in haploidentical donor transplantation recipients and chronic GVHD in unrelated donor transplantation recipients. Although we performed a carefully controlled analysis for patient and disease characteristics, we are not able to adjust for transplantation characteristics (eg, graft type, GVHD prophylaxis) that were confounded by donor type. We acknowledge that the timing of transplantation and donor selection practices vary among transplantation centers, and although we did not identify an effect of transplantation center on survival, there are unknown and unmeasured factors that may have influenced timing and donor choice. A delay in proceeding with haploidentical donor HCT may in part be explained by a preference for a matched unrelated donor and delays incurred with the search process. In addition, universal assessment of myeloid neoplasms using next-generation sequencing (which was not available for most patients in the present study) is likely to offer a more precise assessment of disease risk. Our report also identified 2 major obstacles to a successful outcome after HCT. The results of a recent phase 3 randomized trial suggest lower relapse and higher survival with MAC regimens.24 Therefore, an MAC regimen may be preferred for patients who are able to tolerate such conditions, with less intense regimens reserved for those unable to tolerate myeloablation. Acute and chronic GVHD were high after unrelated donor HCT, and novel approaches to GVHD prophylaxis must be considered. Results of a recently completed phase 3 trial demonstrate lower risk of grade 2 to 4 acute GVHD but not chronic GVHD with the addition of sirolimus to calcineurin inhibitor and mycophenolate GVHD prophylaxis.28 The results of another recent trial show higher GVHD-free, relapse-free survival with tacrolimus, mycophenolate, and PTCY compared with tacrolimus and methotrexate.29 Despite the limitations, the current analysis confirms that HLA-haploidentical donor HCT for MDS in an older population extends access to transplantation with survival and GVHD-free survival comparable to those after HLA-matched unrelated donor HCT. Strategies for better relapse control posttransplantation and reduction in GVHD, especially chronic GVHD, are needed to extend survival for MDS patients.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by public health service grants/cooperative agreements U24-CA076518 from the National Cancer Institute, National Heart, Lung and Blood Institute, and National Institute of Allergy and Infectious Diseases, National Institutes of Health; HHSH250201200016C from the Health Resources and Services Administration; and N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, Department of the Navy, Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Footnotes
Requests for data sharing should be e-mailed to the corresponding author, Michael R. Grunwald (michael.grunwald@atriumhealth.org).
Authorship
Contribution: M.R.G., M.-J.Z., H.E., V.G., T.N., and M.E. designed the research; M.H.J., A.S.M., and M.-J.Z. analyzed the data; and all authors wrote the paper.
Conflict-of-interest disclosure: M.R.G. receives personal fees from AbbVie, Agios, Amgen, Cardinal Health, Bristol-Myers Squibb/Celgene, Daiichi Sankyo, Gilead, Incyte, Karius, Merck, Pfizer, Premier, and Trovagene and research funding from Forma Therapeutics, Genentech/Roche, Incyte, and Janssen. R.N. receives personal fees from Viracor, Magenta, and Kadmon. T.N. receives research support from Novartis and Karyopharm. V.G. receives personal fees from Novartis, Sierra Oncology, Pfizer, and Celgene and research grants from Novartis and Incyte. D.J.W. receives personal fees from FATE Therapeutics and a research grant from Incyte. The remaining authors declare no competing financial interests.
Correspondence: Michael R. Grunwald, Department of Hematologic Oncology and Blood Disorders, Levine Cancer Institute, Atrium Health, 1021 Morehead Medical Dr, LCI Building 2, Suite 60100, Charlotte, NC 28204; e-mail: michael.grunwald@atriumhealth.org.
References
- 1.Robin M, Porcher R, Adès L, et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome: a prospective study on behalf of SFGM-TC and GFM. Leukemia. 2015;29(7):1496-1501. [DOI] [PubMed] [Google Scholar]
- 2.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405-411. [DOI] [PubMed] [Google Scholar]
- 4.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saber W, Cutler CS, Nakamura R, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood. 2013;122(11):1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciurea SO, Shah MV, Saliba RM, et al. Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24(6):1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slade M, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Romee R. Haploidentical hematopoietic cell transplant with post-transplant cyclophosphamide and peripheral blood stem cell grafts in older adults with acute myeloid leukemia or myelodysplastic syndrome. Biol Blood Marrow Transplant. 2017;23(10):1736-1743. [DOI] [PubMed] [Google Scholar]
- 9.Devillier R, Bramanti S, Fürst S, et al. T-replete haploidentical allogeneic transplantation using post-transplantation cyclophosphamide in advanced AML and myelodysplastic syndromes. Bone Marrow Transplant. 2016;51(2):194-198. [DOI] [PubMed] [Google Scholar]
- 10.Robin M, Porcher R, Ciceri F, et al. Haploidentical transplant in patients with myelodysplastic syndrome. Blood Adv. 2017;1(22):1876-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 14.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Committee of the International Bone Marrow Transplant Registry. Consensus among bone marrow transplant for diagnosis, grading and treatment of chronic graft versus host disease. Bone Marrow Transplant. 1989;4(3):247-254. [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 16.Cox DR. Regression models and life tables. J R Stat Soc [Ser A]. 1972;34(4):187-217. [Google Scholar]
- 17.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489-1500. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza A, Fretham C, Lee SJ, et al. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177-e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536-1542. [DOI] [PubMed] [Google Scholar]
- 22.Cogle CR. Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep. 2015;10(3):272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon SR, St Martin A, Shah NN, et al. Myeloablative vs reduced intensity T-cell-replete haploidentical transplantation for hematologic malignancy. Blood Adv. 2019;3(19):2836-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eapen M, Brazauskas R, Hemmer M, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2(16):2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bashey A, Zhang M-J, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide [published correction appears in J Clin Oncol. 2019;37(6):528]. J Clin Oncol. 2017;35(26):3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Wang H-X, Lai Y-R, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30(10):2055-2063. [DOI] [PubMed] [Google Scholar]
- 28.Sandmaier BM, Kornblit B, Storer BE, et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial. Lancet Haematol. 2019;6(8):e409-e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolaños-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil and cyclophosphamide; tacrolimus and bortezomib; or tacrolimus, methotrexate and marovoric versus tacrolimus and methotrexate): a randomized phase 2 trial with a non-randomized contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132-e143. [DOI] [PMC free article] [PubMed] [Google Scholar]