Abstract
Background:
The incidence and area of arbovirus infections is increasing around the world. It is largely linked to the spread of the main arbovirus vectors, invasive mosquito of the genus Aedes. Previously, it has been reported that Aedes aegypti reemerged in Russia after a 50-year absence. Moreover, in 2011, Ae. albopictus was registered in the city of Sochi (South Russia, Black Sea coast) for the first time. In 2013, Asian Ae. koreicus was found in Sochi for the first time.
Methods:
Mosquitoes were collected using the following methods: larvae with a dip net, imago on volunteers and using bait traps. The mosquitoes were identified using both morphology and sequencing of the second internal transcribed spacer of the nuclear ribosomal RNA gene cluster.
Results:
In August 2016, Ae. koreicus larvae and imago and a single male of Ae. aegypti were found on the southern coast of the Crimean Peninsula, where they were not registered before. Newly obtained DNA sequences were registered in GenBank with the accession numbers MF072936 and MF072937.
Conclusion:
Detection of invasive mosquito species (Ae. aegypti and Ae. koreicus) implies the possibility of their area expansion. Intensive surveillance is required at the Crimean Peninsula to evaluate the potential for the introduction of vector-borne diseases.
Keywords: Mosquito monitoring, Arbovirus vectors, Aedes aegypti, Aedes koreicus, DNA
Introduction
At present, the invasive species Aedes (Stegomyia) aegypti, Aedes (Stegomyia) albopictus and Aedes (Hulecoeteomyia) koreicus are spreading across the world and have been registered in Caucasian coast of the Black Sea in southern Russia since 2011 (1–4). All of them are known as vectors of human pathogens (4). Invasive Aedes species have adapted its habitat preferences to human made containers, such as water storage tanks, discarded tires and jars, and water-filled pots (4). Due to the changing climate and the introduction of artificial hatching habitats, it was expected that these mosquito species would also spread to the southern coast of Crimea, which has a climate very similar to Caucasus. In 2010, an entomological survey in the southern coast of Crimea found 15 blood-sucking mosquito species, namely three species of the genus Anopheles (An. claviger, An. maculipennis, An. plumbeus), 3 species of the genus Culex (Cx. hortensis, Cх. pipiens, Cх. territans), five species of the genus Aedes (Ae. refiki, Ae. cantans, Ae. dorsalis, Ae. cataphylla, Ae. geniculatus), three species of the genus Culiseta (Cs. annulata, Cs. longiareolata, Cs. morsitans) and Uranotaenia unguiculata (5). The coastal area of Crimea, especially the southern coast, is densely populated and attracts approximately five million tourists each year. The goal of the present study was to survey the southern coast of Crimea on the presence of invasive mosquito species.
Materials and Methods
Mosquito collection was carried out in August, 2016. Mosquito sampling sites are shown in figure 1. The larvae were collected with a dip net, while the imago were collected either on volunteers using sucking tubes or “Electro-frog” bait traps (LMD-Komplekt Plus, Russia).
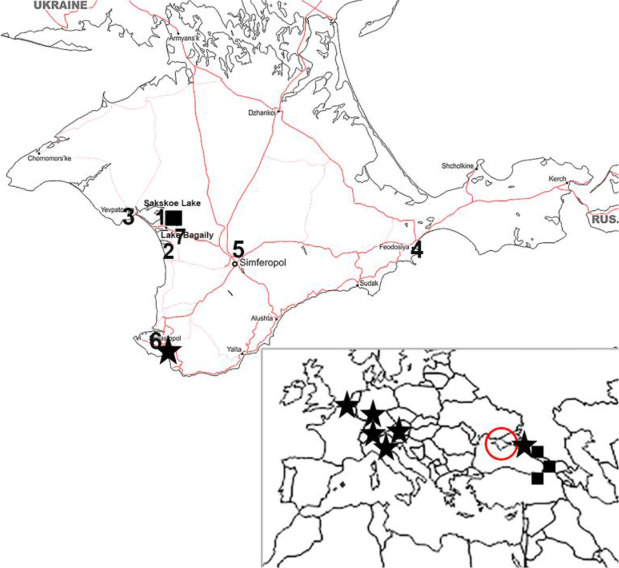
Fig. 1.
Map of Crimean Peninsula with the mosquito sampling locations indicated as in Table 1. The map of Europe with the current known established populations of Aedes koreicus and Ae. aegypti in Europe (ECDC; 2020. Available from: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps ), Crimean Peninsula indicated with a circle. Locations of Ae. aegypti collection are shown with a square, Ae. koreicus - with a star.
Mosquito sampling sites had different conditions. Priboy vacation resort (Number 1 in Table 1, Fig. 1) is located 300–500 meters to the northwest of Sakskoe Lake, one kilometer to the east is the resort city Saki, a town with well-developed residential areas. Frunzenets vacation resort (Number 2 in Table 1, Fig. 1) is located one kilometer away from a village on the shores of Lake Bagaily (Number 7 in Table 1, Fig. 1), which were overgrown with reeds and the inshore depth reached 1 meter. Mosquito larvae were collected in the thickets of the reeds. Departure of imago from the lake was registered. Imagoes were collected in the peak mosquito activity period from 8:30PM to 9:30PM.
Table 1.
Composition of adults and larvae of 661 collected mosquitoes
| Number on the map | Sample site | Coordinates | Mosquito species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. koreicus | Ae. caspius | Cx. pipiens | Cx. modestus | Cx. hortensis | Cq. richiardii | Cs. longiareolata | ||||||
| 1 | Priboy | 45°13′05″N | |||||||||||
| 33°52′22″ E | 1♂ | 3♀ | 154♀ 5♂ | 157♀4♂ | 11♀ | ||||||||
| 2 | Frunzenets | 45°02′37″N | |||||||||||
| 33°63′28″E | 1♀ | 52♀3♂ | 90♀3♂ | 2♀ | |||||||||
| 3 | Evpatoria | 44°12′00″N | |||||||||||
| 33°21′30″E | 26♀ | 21♀ | |||||||||||
| 4 | Koktebel | 44°57′35″N | |||||||||||
| 35°14′52″E | 3♀ | 3♀ | |||||||||||
| 5 | Simferopol | 44°56′53″N | |||||||||||
| 34°06′15″E | 12♀10L | ||||||||||||
| 6 | Sevastopol | 44°43′55″N | |||||||||||
| (Lesnoy | 33°34′55″E | 3♀29L | 2L | 33L | |||||||||
| Village ) | |||||||||||||
| 7 | Lake | 45°02′37″N | |||||||||||
| Bagaily | 33°63′28″E | 8L | 16L | 9L | |||||||||
| Total number of individuals | 1 | 32 | 4 | 275 | 294 | 9 | 13 | 33 | |||||
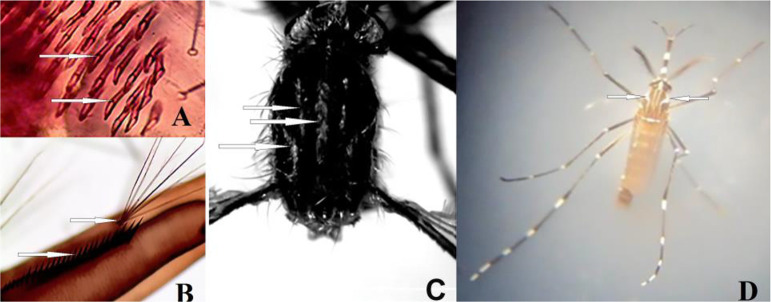
The mosquitoes were identified by morphological key (7) and DNA sequencing. The most reliable characteristics for Ae. koreicus larvae were the form and number of сomb scales on the lateral view of larval abdominal segment (Fig. 2A), position of the siphonal tuft and the number of the pectin teech (Fig. 2B). Imago Ae. koreicus show a black and white pattern due to the presence of white scale patches on a black background and have clear longitudinal lines on the scutum (Fig. 2C). Two narrow white dorsocentral stripes are characteristic for Ae. aegypti (Fig. 2D).
Fig. 2.
Diagnostic characters of Aedes koreicus larva (A, B) and female (C), Ae. aegypti male (D)
DNA extraction was performed using Diatoms DNA prep kit (Isogen, Moscow). The Encyclo PCR kit (Evrogen, Moscow) and primers complementary to 5.8S and 28S rRNA genes (8) were used for amplification of the second internal transcribed spacer (ITS2) region of rRNA genes. Amplification products were visualized in 1% agarose gel and purified from the gel using a clean-up kit (Evrogen, Moscow). Sequencing was performed using ABI PRISM® 310 Genetic Analyzer with BigDye® Terminator kit (Applied Biosystems, USA). The obtained DNA sequences were registered in GenBank (accession numbers MF072936 and MF072937). The internet resource https://www.gismeteo.ru/diary/ was used for climate analysis.
Results
Imago and larvae of eight mosquito species, 661 individuals in total, were collected on the Crimean Black Sea coast and in Simferopol (Fig. 1, Table 1).
In Simferopol, Cx. pipiens was collected in water storage tanks. In Lesnoy Village (№6 in Table 1, Fig. 1), located close to the town of Sevastopol, mosquitoes were collected in an artificial concrete basin with an area of 23m2 and a depth of 0.5m. In this artificial basin, the larval density per 1m2 was the highest: Ae. koreicus – 238 individuals; Cs. longiareolata – 256; and Cx. pipiens – 17.
Among them, one male Ae. aegypti was discovered at Priboy vacation resort. Morphological identification was confirmed by DNA sequencing. This Ae. aegypti ITS2 DNA sequence was deposited to GenBank (accession number MF072936). The ITS2 nucleotide sequence of the Crimean Ae. aegypti was identical to the one from Abkhazia, 2012 (MH142316) and Adler (Russia), 2013 (MH142319). There was one nucleotide substitution in ITS2 (99% identity) between Ae. aegypti found in Crimea (MF072936) and Ae. aegypti sequences registered in GenBank: Rockefeller strain KF471581-KF471583; JX423805 and JX423807 from Saudi Arabia; KU497616 from China; and KF471579 from New Caledonia.
Larvae of the species Ae. koreicus were found close to Sevastopol in Lesnoy Village (Table 1), in an artificial concrete basin. Aedes koreicus imago were found next to this pond. The sequence of the ITS2 genome region of Ae. koreicus larvae found in Crimea was identical to the DNA sequence of Ae. koreicus from Belgium (KF471636 and JF430391) and Sochi (HG763830). The Crimean Ae. koreicus DNA sequence was deposited in GenBank with the accession number MF072937.
In addition to these two invasive species, representatives of six autochthonous mosquito species were registered in the studied area. Imagoes sampled at all locations were mainly represented by the species Cx. pipiens, Cx. modestus, and a few individual Coquillettidia richiardii and Ae. caspius (Table 1). Culiseta longiareolata and Cx. pipiens larvae were found in Lesnoy Village (Table 1).
Discussion
Screening of mosquitos on the south coast of Crimean Peninsula found, among others, Ae. aegypti and Ae. koreicus, which have not been registered in Crimea before 2016 (5). In 2018, Kovalenko and Tikhonov (9) examined eight regions of the Crimea and collected seven Ae. koreicus females in Sevastopol and three in Simferopol. Although no Ae. koreicus larvae or breeding sites were found in this study, this data implied the spread of mosquitoes to the center of the peninsula. So, additional finding of Ae. koreicus imagoes in Crimea in 2018 confirms the establishment of Ae. koreicus population in Crimea.
Native areas of Ae. koreicus are Korean peninsula, China, Japan, and the Russian Far East. Recently, Ae. koreicus has been expanding to new areas due to exobiological plasticity. The species Ae. koreicus was first found in the European part of Russia in 2013 in the center of the town of Sochi (4, 9, 10). At present, Ae. koreicus has been registered in many European countries including Belgium, northern Italy, Hungary, Slovenia and Germany (11–18). It is known that Ae. koreicus can withstand lower winter temperatures and is drawn to populated areas located near woods. Its larvae, as well as those of Ae. aegypti and Ae. аlbopictus, develop in artificial basins. Females usually attack in the open air, occasionally indoors. Mosquitoes overwinter in the egg stage. Aedes koreicus has shown vector competence in the field and in laboratory experiments for a number of pathogens including dengue, West Nile fever, Japanese encephalitis viruses and Dirofilaria (12, 19). Therefore, discovery of Ae. koreicus in Crimea marks a significant threat of the introduction and spread of vector-borne diseases.
Detection of a single Ae. aegypti male can be attributed to either breeding in the close-by residential area, which provides artificial reservoirs serving as breeding habitats, or an isolated introduction, possibly from the Black Sea coast of the Caucasian region, where these mosquitoes were recently registered (2, 4, 6). The latter possibility has been exemplified previously by discovery of a single Ae. aegypti male in Northwest England (53°30′42″N, 2°59′01″W) in 2014, where climate, temperature and humidity were generally unsuitable for this new invasive mosquito (20). In Crimea, where the Ae. aegypti male was discovered in the present study, the highest temperature during the summer season was registered in August 2016. At the time of mosquito collection, the temperature reached 36 °C during daylight hours and 28–31 °C at night. The relative humidity in July and August (67–68%) was uncomfortable for these mosquitoes. During May–August, it rained for only 2 days and the total amount of precipitation over the year was approximately 400mm whereas in Sochi, where Ae. aegypti mosquitoes were previously registered, the annual rainfall exceeds 1500 mm. It is believed that Ae. aegypti distribution is limited to areas with a January isotherm of 10 °C and mean annual temperatures of 15 °C, whereas in Crimea in the winter 2015–2016 the lowest winter temperature reached −15 °C, and in subsequent years it was between −6 °C and −7 °C. Unfavorable conditions may indicate that Ae. aegypti was an occasional introduction to the peninsula. To our knowledge, there is no published data on findings of Ae. aegypti in Crimea (https://ecdc.europa.eu, 2018). At the same time, Ae. aegypti is well established on the Black Sea coast in Caucasus (4), and is now spreading along the Black Sea coast of Turkey and Georgia (6, 21).
In 2014, we suggested that Ae. albopictus mosquitoes were likely to appear in Crimea (22), but neither larvae nor imago of this species were discovered during the present study. Nevertheless, climate change and the detection of the more cold - sensitive Ae. aegypti highlights the risk of Ae. albopictus rooting in Crimea and calls for detailed entomological monitoring on the peninsula. The predominance of Сх. pipiens and Cx. modestus imagoes can be explained by the proximity of the sampling areas Frunzenets and Priboy to lakes (Fig. 1), which are a favorable environment for the development of the genus Сulex larvae, as evidenced by the presence of Culex hortensis larvae at Bagaily Lake (Table 1). The absence of Cx. hortensis imago among the collected samples (as compared with the larvae samples) is due to the fact that these species are reluctant to attack humans and spend most of the time in caves and similar sheltered environments.
Conclusion
In the current study, larvae breeding habitats for Ae. koreicus and Ae. aegypti imago have been found on the southern coast of the Crimean Peninsula for the first time. The Ae. aegypti species was represented by a single male specimen; therefore, it is not clear if it has yet been established in Crimea. Our study indicates that Ae. koreicus is locally established in Crimea and its northward expansion is expected, highlighting the challenges for vector surveillance and control programs.
Acknowledgements
This work was supported by the RFBR grants No.16-04-00091 and No.19-04-00739. We are grateful to “LMD-Komplekt Plus” company, namely to A.S. Poznyshev, S.I. Kozhurin and R.A. Mingaliev, for providing Electrofrog traps for mosquito collection. We declare that we have no conflict of interest.
References
- 1. Ganushkina LA, Tanygina EYu, Bezzhonova OV, Sergiev VP. (2012) Detection of Aedes Stegomyia) albopictus Skuse Mosquitoes in the Russian Federation. Med Parazitol (Mosk). (1): 3– 4. [PubMed] [Google Scholar]
- 2. Ganushkina LA, Bezzhonova OV, Patraman IV Tanygina EIu, Sergiev VP. (2013) Distribution of Aedes Stegomyia aegypti and Aedes Stegomyia albopictus Skuse mosquitos on the Black Sea coast of the Caucasus. Med Parazitol (Mosk). (1): 45– 46. [PubMed] [Google Scholar]
- 3. Bezzhonova OV, Patraman IV, Ganushkina LA, Vishmirskii OI, Sergiev VP. (2014) The first finding of the invasive species Aedes (Finlaya) koreicus Edwards, 1917 in European Russia. Med Parazitol (Mosk). (1): 16– 19. [PubMed] [Google Scholar]
- 4. Ganushkina LA, Patraman IV, Rezza G, Migliorini L, Litvinov SK, Sergiev VP. (2016) Detection of Aedes aegypti, Aedes albopictus, and Aedes koreicus in the Area of Sochi, Russia. Vector Borne Zoonotic Dis. (1): 58– 60. [DOI] [PubMed] [Google Scholar]
- 5. Razumeiko VN, Ivashov AV, Oberemok VV. (2010) Seasonal activity and dynamics of density of blood-sucking mosquitoes (Diptera, Culicidae) in the reservoirs of the south coast of Crimea. Scientific notes of V. I. Vernadsky Taurida National University Series “Biology, Chemistry”. 23 (3): 114– 128. [Google Scholar]
- 6. Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. (2016) Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue and Zika Outbreaks in Europe. PLoS Negl Trop Dis. 10( 4): e0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutsevich AV, Monchadskii AS, Shtakelberg AA. (1970) Fauna of the USSR. Diptera. Mosquitoes. Vol. 3 (4). Nauka, Leningrad. [Google Scholar]
- 8. Porter CH, Collins FH. (1991) Species-diagnostic differences inaribosomal DNA internal transcribed spacer from the sibling species Anopheles freeborni and Anopheles hermsi Diptera: Culicidae. Am J Trop Med Hyg. 45 (2): 271– 279. [DOI] [PubMed] [Google Scholar]
- 9. Kovalenko IS, Tikhonov SN. (2019) Recording of Aedes koreicus (Edwards, 1917) (Diptera, Culicidae) in the territory of Crimea. Entmol. Rev. 99: 388– 392. [Google Scholar]
- 10. Fedorova MV, Ryabova TE, Shaposhnikova LI, Lopatina YuV, Sebenzova AN, Yunicheva YuV. (2017) Invasive species of mosquitoes on the territory of Sochi: larval development place sand counting methods. Med Parazitol (Mosk). 4: 3– 9. [Google Scholar]
- 11. Deblauwe I, Sohier C, Schaffner F, Rako- toarivony LM. (2014) Implementation of surveillance of invasive mosquitoes in Belgium according to the ecdc guidelines. Parasit Vectors. 7: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Capelli G, Drago A, Martini S, Montarsi F, Soppelsa M, Delai N, Ravagnan S, Mazzon L, Schaffner F, Mathis A, Luca MD, Romi R, Russo F. (2011) First report in Italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasit Vectors. 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montarsi F, Martini S, Pont M D, Delai N, Milone NF, Mazzucato M, Soppelsa F, Cazzola L, Cazzin S, Ravagnan S, Ciocchetta S, Russo F, Capelli G. (2013) Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in North-Eastern Italy. Parasit Vectors. 6: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suter T, Flacio E, Fariña BF, Engeler L, Tonolla M, Müller P. (2015) First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasit Vectors. 8: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcantonio M, Metz M, Baldacchino F, Arnoldi D, Montarsi F, Capelli G, Carlin S, Neteler M, Rizzoli A. (2016) First assessment of potential distribution and dispersal capacity of the emerging invasive mosquito Aedes koreicus in Northeast Italy. Parasit Vectors. 9: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurucz K, Kiss V, Zana B, Schmieder V, Kepner A, Jakab F, Kemenesi G. (2016) Emergence of Aedes koreicus Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitol Res. 115 (12): 4687– 4689. [DOI] [PubMed] [Google Scholar]
- 17. Kalan K, Šušnjar J, Ivović V, Buzan E. (2017) First record of Aedes koreicus Diptera, Culicidae in Slovenia. Parasitol Res. 116 (8): 2355– 2358. [DOI] [PubMed] [Google Scholar]
- 18. Werner D, Zielke DE, Kampen H. (2016) First record of Aedes koreicus Diptera: Culicidae in Germany. Parasitol Res. 115 (3): 1331– 1334. [DOI] [PubMed] [Google Scholar]
- 19. Schneider J, Valentini A, Dejean T, Montarsi F, Taberlet P, Glaizot O, Fumagalli L. (2016) Detection of invasive mosquito vectors using environmental DNA (eDNA) from Water Samples. PLoS One. 11( 9): e0162493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dallimore T, Hunter T, Medlock JM, Vaux AG, Harbach RE, Strode C. (2017) Discovery of a single male Aedes aegypti (L.) in Merseyside, England. Parasit Vectors. 10( 1): 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotsakiozi P, Gloria-Soria A, Schaffner F, Robert V, Powell JR. (2018) Aedes aegypti in the Black Sea: recent introduction or ancient remnant? Parasit Vectors. 11 (1): 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganushkina LA, Morozovа LF, Patraman IV, Sergiev VP. (2014) Assessment of the risk expansion of the habitans of the mosquitos Aedes aegypti L. and Aedes albopictus Scuse in Russia. Med Parazitol (Mosk). (4): 8– 11. [PubMed] [Google Scholar]