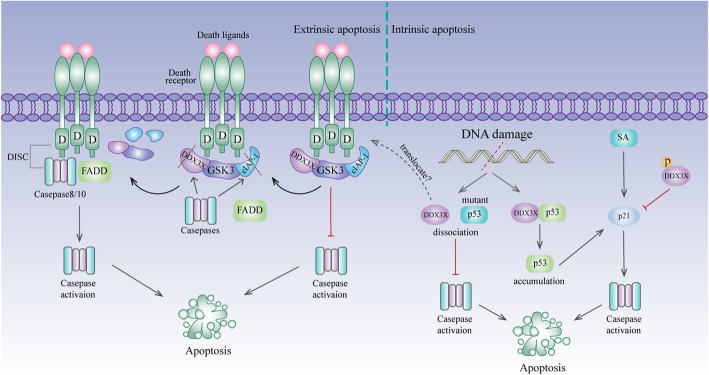
Fig. 4.
DDX3X and apoptosis. In the extrinsic apoptosis pathway, DDX3X binds with GSK3 and cIAP-1, forming an anti-apoptotic complex to cap major death receptors. After death ligands bind to their receptors, the anti-apoptotic complex is destroyed by inactivation of GSK3β and cleavage of DDX3X and cIAP-1 by caspases. Cleavage occurred in the N-terminus of DDX3X, and the truncated protein can still bind GSK3-β. In the intrinsic apoptosis pathway, DDX3X binds wild-type P53 and mutant P53 in tumours. When encountering DNA damage, DDX3X binds wild-type P53 and stabilizes its protein level, promoting P53/P21 axis-mediated apoptosis. However, DDX3X and mutant P53 are separated after DNA damage occurs, which impedes Caspase activation in P53-mutant tumours. This disassociation may contribute to the translocation of DDX3X to death receptors. However, in HeLa cells, phosphorylated DDX3X reverses sanguinarine (SA)-induced intrinsic apoptosis by strongly repressing P21 expression