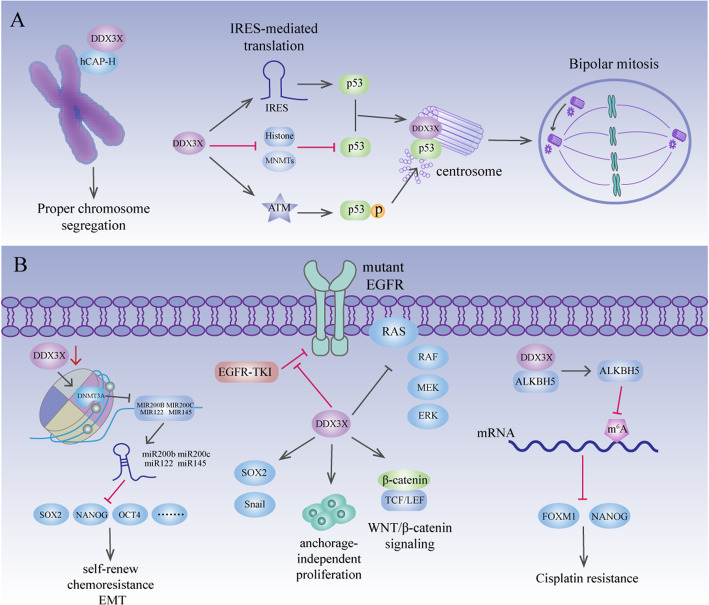
Fig. 8.
DDX3X in mitosis and stemness. a During prophase/prometaphase of mitosis, DDX3X translocates in close proximity to the condensing chromosomes and interacts with hCAP-H to promote chromosome segregation. In colon cancer and osteosarcoma, DDX3X upregulates P53 expression by promoting IRES-mediated translation of P53, thus preventing DNMTs from hypermethylating the P53 promoter and repressing the binding of repressive histone markers to the P53 promoter. In addition, DDX3X activates ATM kinase to phosphorylate P53, which leads to the localization of P53 to centrosomes. At the centrosome, DDX3X interacts with P53, leading to inactivation and coalescence of excess centrosomes. b In liver cancer, DDX3X upregulates the expression of miR-200b, miR-200c, miR-122 and miR-145 by reducing DNMT3A binding and hypermethylation of their promoter regions. This subset of miRNAs suppresses the expression of signature stemness genes, including SOX2, NANOG, and OCT4, which are responsible for self-renewal, chemoresistance and EMT. In lung adenocarcinoma cells harbouring an EGFR mutation, DDX3X overexpression induces increased Sox2 and Snail expression, anchorage-independent proliferation, resistance to EGFR-TKIs and promotion of Wnt/β-catenin signalling. In cisplatin-resistant OSCC cells, DDX3X interacts with ALKBH5 and increases its expression. ALKBH5 then upregulates FOXM1 and NANOG expression by demethylating their methylated mRNAs, which eventually leads to cisplatin resistance