Fig. 12.
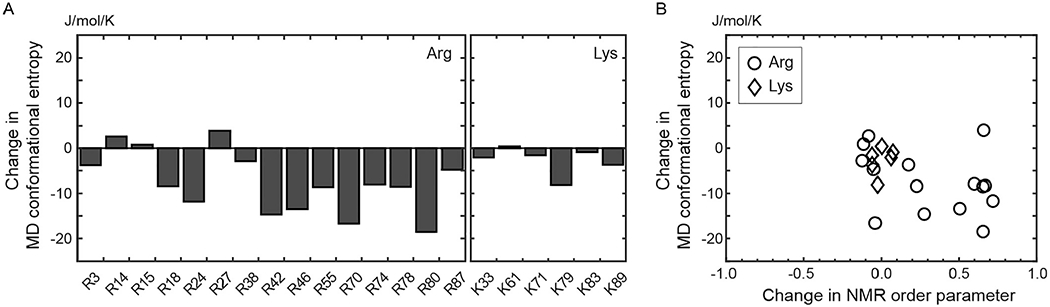
Changes in side-chain conformational entropy for the Lys and Arg side chains of the Egr-1 zinc-finger protein upon binding to the target DNA. (A) Changes in the side-chain conformational entropy of Lys and Arg side chains calculated from 0.6-μs MD trajectories for the free protein and the complex. (B) Correlation between changes in the NMR order parameters and changes in the MD conformational entropy upon complex formation.
