Figure 1. Testing of MSK-DA01 prior to preclinical efficacy and safety studies.
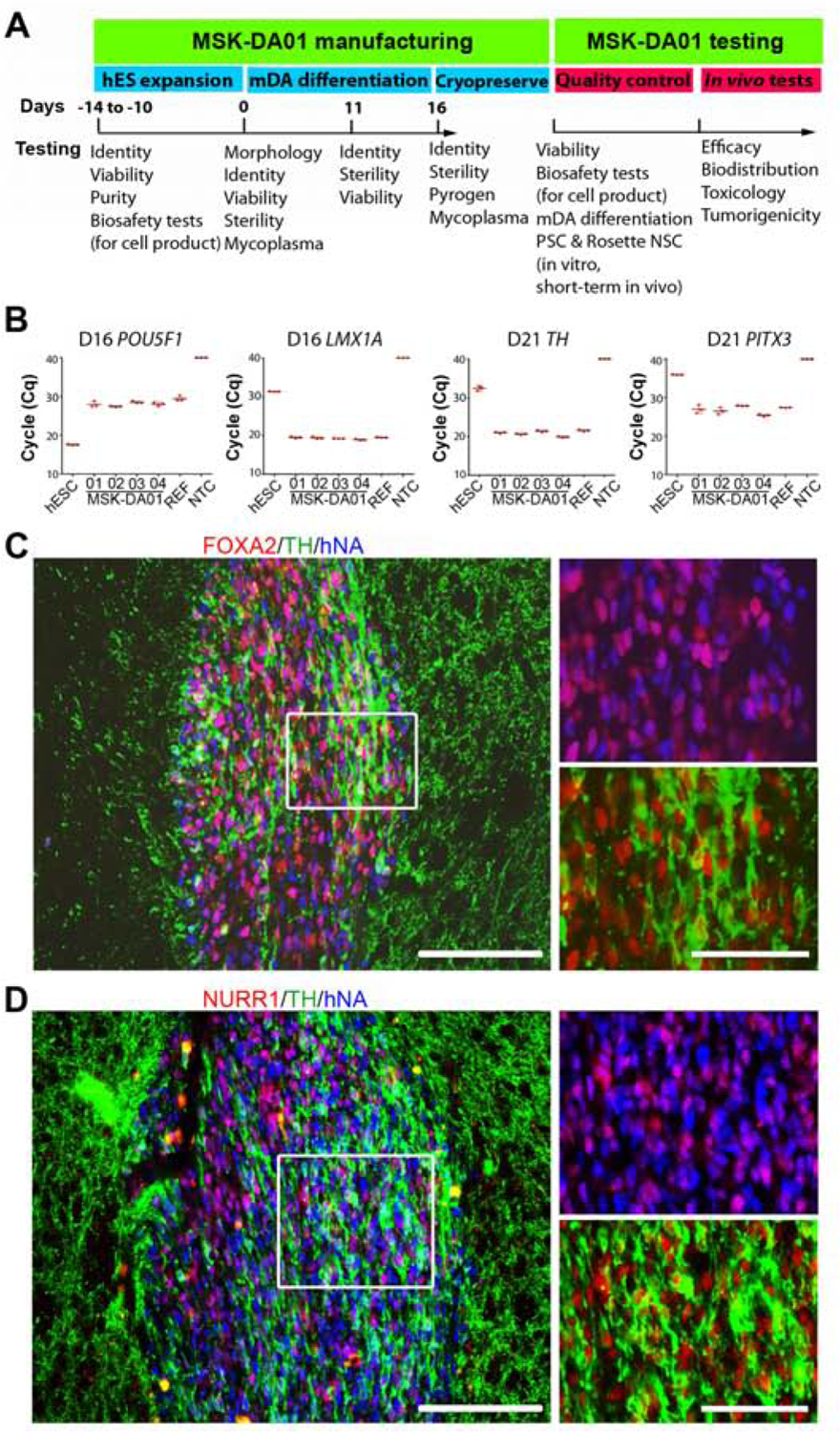
(A) Schematic diagram showing testing undertaken at different steps of manufacture and application of MSK-DA01. (B) Cycle quantification (Cq) values of POU5F1, LMX1A, PITX3 and TH in four lots (01–04) of cryopreserved MSK-DA01 assessed by qRT-PCR. PITX3 and TH were assessed at five days post thaw. WA09 (ES), reference (REF, a previous successful batch) and negative control (NTC, no template control) were included in the analysis. Data are represented as mean±SD (n=3/group). (C) Representative section through a graft 3 weeks post transplantation, immunostained for FOXA2, TH and human nuclear antigen (hNA). (D) Graft immunohistochemistry for NURR1, TH and human nuclear antigen (hNA) showing co-expression in most human cells. Rectangles indicate areas of magnification shown in the right panels. Scale bars=100 µm in left panel and 50 µm in right panels.
