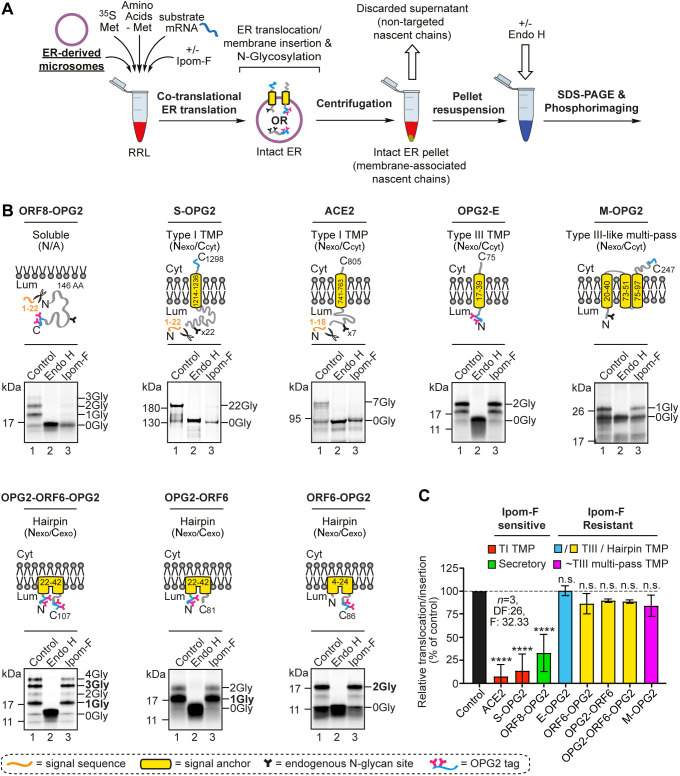
Fig. 2.
Ipom-F selectively inhibits the ER membrane translocation of SARS-CoV-2 proteins. (A) Schematic of in vitro ER import assay using pancreatic microsomes. Following translation, fully translocated or membrane-inserted radiolabelled precursor proteins are recovered and analysed by SDS–PAGE and phosphorimaging. N-glycosylated species were confirmed by treatment with endoglycosidase H (Endo H). (B) Protein precursors of the human angiotensin-converting enzyme 2 (ACE2) and OPG2-tagged versions of the SARS-CoV-2 ORF8 (ORF8–OPG2), spike (S–OPG2), envelope (OPG2–E), membrane (M–OPG2) and ORF6 (a doubly OPG2-tagged version, OPG2–ORF6–OPG2, and two singly OPG2-tagged forms, OPG2–ORF6 and ORF6–OPG2, with predominant N-glycosylated species in bold) were synthesised in rabbit reticulocyte lysate (RRL) supplemented with ER microsomes without or with Ipom-F (lanes 1 and 3). Phosphorimages of membrane-associated products resolved by SDS–PAGE with representative substrate outlines are shown. N-glycosylation was used to measure the efficiency of membrane translocation or insertion, and N-glycosylated (xGly) versus non-N-glycosylated (0Gly) species identified using Endo H (see lane 2). (C) The relative efficiency of membrane translocation or insertion in the presence of Ipom-F was calculated using the ratio of N-glycosylated protein to non-glycosylated protein, relative to the DMSO-treated control (set to 100% efficiency, dashed line). Quantitations are given as mean±s.e.m. for independent translation reactions performed in triplicate (n=3), and statistical significance (one-way ANOVA, DF and F values shown in the figure) was determined using Dunnett's multiple comparisons test. ****P<0.0001; n.s., non-significant P>0.1. AA, amino acids; Cyt, cytoplasm; Lum, lumen; TI, type I; TIII, type III.