Abstract
In this study, we report the isolation and identification of an endophytic strain of Burkholderia cepacia (COPS strain) associated with Polygala paniculata roots. Polygala plants are rich sources of promising microbiomes, of which the literature reports several pharmacological effects, such as trypanocidal, antinociceptive, anesthetic, anxiolytics, and anticonvulsant activities. B. cepacia COPS belongs to a new sequence type (ST 1870) and harbors a genome estimated in 8.3 Mbp which exhibits the aminoglycosides and beta-lactams resistance genes aph(3′)-IIa and blaTEM-116, respectively. Analysis performed using MLST, average nucleotide identity, and digital DNA-DNA hybridization support its species-level identification and reveals its novel housekeeping genes alleles gyrB, lepA, and phaC. The root endophyte B. cepacia COPS drew our attention from a group of 14 bacterial isolates during the primary screening for being potentially active against Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Micrococcus luteus ATCC 9341, Escherichia coli ATCC 25922, and Candida albicans ATCC 10231 and exhibited the broad-spectrum activity against phytopathogenic fungi. In addition, COPS strain showed production of protease, lipase, and esterase in solid media, and its natural product extract showed potent inhibition against fungal plant pathogens, such as Moniliophthora perniciosa, whose antagonism index (89.32%) exceeded the positive control (74.17%), whereas Sclerotinia sclerotiorum and Ceratocystis paradoxa showed high percentages of inhibition (85.53% and 82.69%, respectively). COPS crude extract also significantly inhibited S. epidermidis ATCC 35984, E. faecium ATCC 700221 (MIC values of 32 μg/mL for both), E. faecalis ATCC 29212 (64 μg/mL), and S. aureus ATCC 25923 (128 μg/mL). We observed moderate antagonistic activity against A. baumannii ATCC 19606 and E. coli ATCC 25922 (both at 512 μg/mL), as well as potent cytotoxic effects on Leishmania infantum and Leishmania major promastigote forms with 78.25% and 57.30% inhibition. In conclusion, this study presents for the first time the isolation of an endophytic B. cepacia strain associated with P. paniculata and enough evidence that these plants may be considered a rich source of microbes for the fight against neglected diseases.
1. Introduction
Plant tissues represent a significant source of natural substances for pharmaceutical and biotechnological interest. Drug discovery has been based on medicinal plants for centuries [1–3]. However, endophytes are capable to biosynthesize a plethora of natural products and compounds which are originally believed to be produced only by their host plants [4, 5], and therefore, they are considered alternative suppliers of characteristic phytochemical compounds and represent a vast unexplored reservoir of unique chemical structures [6]. These plant-symbiont microorganisms that live in intimate interaction establish a mutualistic interaction with the host plant by exchanging nutrients and protection; they produce antibiotics and other substances that can protect the plant against stress conditions such as attack by herbivores, pests, and plant pathogens without causing apparent disease symptoms [7–9].
Polygala paniculata (commonly known as “mimosa,” “barba-de-bode,” “barba-de-São-João,” and “vassourinha branca”) is a medicinal plant that frequently grows on the Brazilian coast and is used in traditional medicine due to their analgesic properties and treatment of inflammatory diseases such as asthma, bronchitis, arthritis, and disorders of the kidney [10–12]. However, plants within this genus are well-known producers of a variety of phytochemical compounds [13], such as methyl salicylate, alkaloids [14], xanthones [15, 16], saponins [17, 18], coumarins [11, 19], and styrylpyrones [11]. Natural products extracted from Polygala species are widely studied [13], and numerous reports describe pharmacological effects for their crude extracts such as anti-inflammatory [20, 21], anxiolytic [22], antidepressant [11], trypanocidal [23], antinociceptive [24], neuroprotective [25, 26], antiatherosclerosis [27], antitumor [28, 29], and antifungal [30]. However, the potential of endophytes and rhizospheric-associated microorganisms within these plant genuses remains unknown. Consequently, the exploitation of medicinal plants' microbiome, which produces bioactive metabolites, is fundamental [31, 32].
The introduction of antibiotics enabled the development of therapies for previously incurable diseases. However, resistance to this class of medicines happens faster than the human capability of discovering new compounds and introducing them into clinical practice. Moreover, synthetic approaches to antibiotic production have not been effective enough to completely replace this platform [31, 33, 34].
Likewise, phytopathogenic fungi represent a severe threat to several crops, thus affecting production and quality. Modern agriculture is entirely dependent on agrochemicals; although they can improve crop yield, quality, and shelf-life, they negatively affect the environment and human health. In this regard, issues related to sustainability and practices in defense of the environment have drawn considerable attention [35–37].
Approximately, 13 million people suffer from parasitic diseases caused by Leishmania protozoa infection. Parasite resistance and host toxicity of currently available drugs are a reality and a concern mainly in subtropical countries [38, 39]. On the other hand, microbial resistance to antibiotics has been rapidly spread, is responsible for 33,000 deaths in Europe, and became a concern to public health [40].
We aimed to explore the endophytes of Polygala paniculata and isolate antibiotic- and biotechnology-related enzymes-producing microorganisms. Herein, we present the Burkholderia cepacia COPS, a sequence-type (ST) 1870 strain isolated from P. paniculata roots collected in the Brazilian Atlantic Forest. In addition to its draft-genome, we presented the COPS enzymes and antimicrobial activities.
Burkholderia spp. consist of emerging sources of a plethora and diverse natural products potentially relevant for therapeutic/medicine, biotechnological, and agriculture applications [41]. This genus is a versatile producer of antimicrobial compounds and enzymes and exhibits plant growth-promoting properties. We can find such Gram-negative bacteria in several habitats, ranging from humans (as pathogens) to plants (as endophytes) [42–48].
Although widely used for bacterial systematics, the taxonomic identification by 16S rRNA coding gene among Burkholderia cepacia complex (Bcc) is limited and difficult [49–53]. According to Bach et al. [52], the identities of 16S rRNA and recA genes within Bcc can reach 100% and 95%, respectively. Several studies report the use of housekeeping genes established in multilocus sequence typing (MLST) to differentiate the Bcc species [54–61]. The multilocus sequence analysis (MLSA) of the housekeeping genes atpD, gltB, gyrB, recA, lepA, phaC, and trpB enable an accurate investigation of evolutionary characteristics and high resolution at the species level [56, 57, 61].
2. Materials and Methods
2.1. Biological Material
Polygala paniculata plants samples and their rhizospheric soil were collected in Peruíbe, south coastal municipality of São Paulo State, Brazil (−24° 19 ′12 ″, 46° 59′ 54″) (SisGen, registration number: AF1A75A), and vouchers (F.P.N. Cruz 3) were deposited at the Federal University of Sao Carlos (SPSC) and Botanical Garden of Rio de Janeiro (RB) herbaria and registered to the Brazilian Genetic Resources Managing System (SisGen; registration AF1A75A). Plant's identity was confirmed as Polygala paniculata Linnaeus [62], in accordance with Marques and Gomes [63]. The bacterial community was isolated after superficial disinfection of the plant by serial washing in 70% ethanol for 2 min, NaClO for 3 min, 70% ethanol for 1 min, and double rinse with distilled H2O. The plant structures were grounded and incubated in phosphate-buffered solution (PBS) (NaCl, 8.0 g/L; KCl, 0.2 g/L; Na2HPO4, 1.44 g/L; KH2PO4, 0.24 g/L; pH, 7.4) at 28°C/200 rpm for 2 hours. Subsequently, 100 μL of 1 : 10 serial dilutions were inoculated on tryptic soy agar (TSA) supplemented with benomyl (50 μg/mL) and incubated at 28°C until growth [64, 65]. The isolation of the rhizospheric community was based on Andreote et al. [66] with slight modifications. Ten grams of rhizospheric soil were placed in Erlenmeyer flasks containing 90 mL of sterile PBS and incubated under the same conditions already described. Finally, 100 μL of decimal dilutions was inoculated in TSA and cultured at 28°C until bacterial growth.
2.2. Screening of Antimicrobial and Enzymatic Activities
Fourteen bacterial isolates were randomly selected (four from rhizosphere and ten endophytes) and qualitatively tested by the overlay test [44, 67, 68] for the antimicrobial activity screening. A total of 100 μL of precultured isolates in International Streptomyces Project medium 2 (ISP2) (malt extract: 10 g/L; yeast extract: 4 g/L; and glucose: 4 g/L) [69] was adjusted to OD600 between 0.3 and 0.6 and inoculated in the center of the Petri dishes containing ISP2 agar and incubated at 28°C for 72 hours. Then, the isolates were exposed to chloroform for inactivation, followed by a 30-minute evaporation step. Subsequently, semisolid brain-heart infusion agar (BHI), previously inoculated with test microorganisms (Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 11775, and Candida albicans ATCC 10231), was poured onto the inactivated isolate.
The antagonism test for phytopathogenic fungi was performed as described by Quiroga et al. [70]. The isolated strains were streaked in potato dextrose agar (PDA) at the edges of the plates and incubated at 28°C until their complete growth. Next, a 0.6 cm plug of the phytopathogenic fungus mycelium was placed at the top of the plate containing the grown isolate. The plates were incubated again at 28°C. As a negative control, each phytopathogen was cultured in PDA for indicating the time of the inhibition evaluation [71]. All tests were performed in triplicates. All pathogenic strains used in this study are listed in Table 1.
Table 1.
Pathogenic strains used in this study.
| Public health pathogens | Phytopathogens |
|---|---|
| Staphylococcus aureus ATCC 25923 | Sclerotinia sclerotiorum |
| Staphylococcus aureus ATCC 29213 | Moniliophthora perniciosa |
| Staphylococcus epidermidis ATCC 35984 | Fusarium solani |
| Enterococcus faecium ATCC 700221 | Fusarium oxysporum ATCC 2163 |
| Enterococcus faecalis ATCC 29212 | Sphaceloma sp. |
| Micrococcus luteus ATCC 9341 | Ceratocystis paradoxa |
| Acinetobacter baumannii ATCC 19606 | Alternaria alternata |
| Escherichia coli ATCC 25922 | Fusarium proliferatum |
| Klebsiella pneumoniae ATCC 700603 | Colletotrichum sp. |
| Pseudomonas aeruginosa ATCC 27853 | Fusarium verticillioides |
| Candida albicans ATCC 10231 | Fusarium oxysporum—bean |
| Fusarium oxysporum—cotton | |
| Phytophthora sojae | |
| Rhizopus microsporus |
The assessment of enzymatic potential consisted in a preculture of Polygala paniculata-derived bacteria in 3 mL of tryptic soy broth (TSB–KASVI) and incubated for 48–72 hours at 28°C. Then, 2 μL of the culture was transferred to a M9 enzymatical solid medium (200 mL/L of stock solution (64 g/L Na2HPO4.7H2O; 15 g/L KH2PO4; 2.5 g/L NaCl; 5 g/L NH4Cl)); 2 mL/L 1 M MgSO4; 10 g/L; 0.1 mL/L CaCl2 1 M; 15 g/L agar, pH 7.2, with different supplements, depending on the activity to be studied: (1) 0.5% yeast extract and 1% soluble starch for amylase activity; (2) 0.5% yeast extract and 1% carboxymethyl cellulose for cellulase activity; (3) 0.5% yeast extract and 1% pectin, pH 8.0 for pectin-pectate lyase; and (4) 0.5% yeast extract and 1% pectin, pH 5.0 for pectin-polygalacturonase. The lipase/esterase media consisted of peptone, 10 g/L; NaCl, 5 g/L; CaCl2.H2O, 0.1 g/L; agar, 15 g/L; pH 7.4, supplemented with 1% (v/v) of Tween 20 and Tween 80 for lipolytic and esterastic activities, respectively. The following components were used for protease medium: 5 g/L of tryptone; 2.5 g/L of yeast extract; 1.0 g/L of glucose; 2.5 g/L of NaCl; 15 g/L of agar; and the pH adjusted to 7.0. All components were sterilized at 121°C for 15 minutes, and 100 mL skimmed milk was added for completing one liter.
The experiment was performed in triplicate, and the isolates were incubated for 48 h at 28°C. Congo red dye was used as a revealer (15 minutes) followed by a washing step with 5 M NaCl for the cellulase activity visualization. Iodine tincture was used for amylase and pectinases tests. The enzymatic production of protease, lipase, and esterase activities was visualized as a bright halo around the colonies [72].
2.3. Natural Products Extraction
The isolate GLB 2 was selected for the next experiments due to its broad-spectrum, high bioactivity rates in antimicrobial screening, and capacity to biosynthesize multiple enzymes. The natural products extract (NPE) of the isolate GLB 2 was obtained through the inoculation of 10 μL of a preculture in round-bottom tubes (12 mL capacity) containing 3 mL of ISP2 and incubated at 220 rpm/28°C for three days. Subsequently, the culture was inoculated in 100 mL of ISP2 in a 250 mL flask and maintained under the same conditions for seven days. The culture was then centrifuged at 4,500 rpm for 10 min and extracted by solid-phase using polypropylene mesh packages containing 1.5 g of Amberlite® XAD16 resin (Sigma-Aldrich), which were added to the fermented broths and overnight incubated on a rotary shaker under the same conditions described. The resin bags were then removed and packed in glass tubes containing 20 mL of MeOH : EtOAc (1 : 1). The extracts were dried and concentrated by Vacufuge plus (Eppendorf), resuspended at a 50 mg/mL concentration in 100% dimethyl sulfoxide (DMSO) and maintained at −80°C [73].
2.4. Antagonism Index in Phytopathogens
The isolate GLB 2 NPE antagonism index (AI) was determined by the inoculation of 500 μg of its NPE in a sterile disk placed on top of the plate. A plug of phytopathogenic fungus was inoculated in the center. The assay positive control consisted of culturing each phytopathogen, as described above, in the presence of 500 μg of benomyl, whereas for the negative control, each phytopathogen was cultured to indicate the expected fungi growth. All measurements were performed in triplicate (Figure 1).
Figure 1.
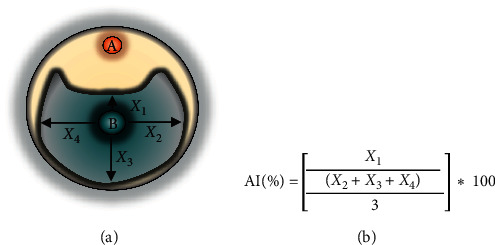
Schematic representation of the quantitative assay for the AI determination: (a) paper disk containing NPE of Burkholderia cepacia COPS; (b) phytopathogenic fungus mycelium. The inhibition index was calculated by the formula using the means of the mycelial growth measurements in centimeters.
2.5. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of the NPE was performed in triplicate, according to recommendations of the Clinical and Laboratory Standards Institute (CLSI) [74] against pathogenic bacteria Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 35984, Enterococcus faecalis ATCC 29212, Enterococcus faecium ATCC 700221, Acinetobacter baumannii ATCC 19606, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa ATCC 27853. An overnight culture of each pathogen was diluted until reaching a 5 × 105 CFU.mL−1 final concentration in Müeller–Hinton cation adjusted (MHCA) broth with several concentrations of COPS NPE (from 100 to 1.5 μg/mL at 1% DMSO) and incubated for 24 h, at 37°C. As a positive control, ciprofloxacin was used at the same concentration gradient, and bacterial suspensions in MHCA broth and 1% DMSO were used as negative controls. The bioactivity was analyzed by measuring each well's optical density 24 hours after administration of NPE in a microtiter plate reader.
2.6. In Vitro Activity Assay against Leishmania spp
Cultures of promastigote forms of Leishmania infantum strain MHOM/BR/1972/LD and L. major, maintained at −80° C in a freezing solution (DMSO/fetal bovine serum—1 : 10), were thawed and transferred to 9 mL of Schneider's medium (Sigma-Aldrich, USA) supplemented with 10% inactivated fetal bovine serum (Vitrocell Embriolife, BRA), 10% human urine from male volunteers aged between 25 and 35 years, and 1% of penicillin and streptomycin. The cultures were then centrifuged for 5 min at 5000 rpm, and the pellet was resuspended in 1 ml of the same medium, which was transferred to a 50 mL capacity cell culture bottle containing 9 mL of fresh medium and incubated at 26°C in 5% CO2.
The toxicity assay consisted of the inoculation of promastigotes in the stationary phase (107 cells/mL) in 96 well plates containing different concentrations of NPE (200, 100, 50, 25, 10, and 1 μg/mL). They were tested in biological triplicates and experimental duplicates. The OD550 was measured by a spectrophotometer (Thermo Scientific Multiskan GO spectrophotometer) 24 hours after the administration of NPE. Amphotericin B (Sigma-Aldrich, USA) at 100 μM was used as a positive control. The cell viability percentage was calculated from the absorbance of the negative control, which represents 100% of cell viability (% of living cells = test OD550 × 100/negative control OD550), and IC50 was measured by nonlinear regressions of the values found for each concentration in, at least, three independent experiments.
2.7. Statistical Analysis
The results were analyzed by GraphPad Prism 8.0.1 software (San Diego, California, USA), and the Shapiro–Wilk test was applied to all data obtained. Subsequently, one-way ANOVA (one-way analysis of variance), followed by Dunnett's multiple comparisons test were applied using a statistical significance at p < 0.05 (95%).
The statistical significance in Leishmania assays was calculated by Tukey's multiple comparison test after one-way ANOVA analysis.
2.8. Genomic DNA Isolation
Isolate GLB 2 was cultivated for three days/28°C in 20 mL of ISP2 broth (malt extract, 10 g/L; glucose, 4 g/L; yeast extract, 4 g/L; pH 7,3), and cells were harvested by centrifugation at 8000 rpm for 10 minutes. Genomic DNA was then extracted using DNeasy Kit (Qiagen) with a lysis process consisting of four cycles of incubation of cell pellets at 65°C for 15 minutes in 180 μL of ATL buffer followed by freezing at −80°C for 15 minutes. DNA was eluted in 100 μL of H2O, and its quality was analyzed on 0.7% agarose gel stained with hydra green and quantified using a NanoDrop.
2.9. Genome Sequencing, Assembly, and Functional Annotations
Pair-ended sequences were obtained by Illumina MiSeq (Illumina, San Diego, USA) platform using a 2x250 bp library prepared using Nextera XT DNA kit with v3 600 cycles. Illumina paired end reads were first preprocessed for quality analysis using FastQC—Unipro UGENE v. 34 [75] and trimmed using Trimmomatic—Galaxy v. 0.38.0 [76] for removing quality bases lower than 20 and adapters. Burkholderia sp. genome was assembled using SPAdes Genome Assembler—Galaxy v. 3.12.0 [77] applying kmers 21, 33, and 55. Quality assessment of assemblies was evaluated using the software QUAST Genome assembly Quality—Galaxy v. 5.0.2 [78] followed by annotation using Prokka—Prokaryotic genome annotation v. 1.14.1—Galaxy v. 1.14.5 [79] and Rast v. 2.0 [80] (https://rast.nmpdr.org/rast.cgi). Finally, GLB 2 genomic contigs were mapped against the reference genome of B. cepacia ATCC 25416 using CONTIGuator v. 2.7.4 [81].
2.10. Phylogenetic and Multilocus Sequence Analysis
Isolate GLB 2 16S ribosomal RNA gene was identified by RNAmmer 1.2 server (http://www.cbs.dtu.dk/services/) [82] and identified based on 16s rRNA BLAST search.
The complete 16S rRNA and housekeeping gene sequences atpD (ATP synthase β chain—1,395 bp), gltB (glutamate synthase large subunit—4,704 bp), gyrB (DNA gyrase B—2,475 bp), recA (recombinase A—1,071 bp), lepA (GTP binding protein—1,794 bp), phaC (acetoacetyl-CoA reductase—741 bp), and trpB (tryptophan synthase subunit B—1,194 bp) from reference Bcc members were retrieved from PubMLST database (http://www.pubMLST.org/bcc/) and aligned with the endophytic B. cepacia nucleotide sequences by ClustalW and concatenated using MEGA X software [83].
The phylogenetic trees of multiple alignments of 16S rRNA and concatenated housekeeping gene sequences were generated by MEGA X. The neighbor-joining method [84, 85] using Jukes-Cantor as a substitution model, respectively, as well 1000 bootstrap replications as branch support for the construction of the trees.
2.11. Comparative Genomic Analysis
Average nucleotide identity (ANI) [86–88], calculation of tetra nucleotide frequencies, and correlation coefficients [89] values were estimated based on BLAST alignments using JSpeciesWS (http://jspecies.ribohost.com/jspeciesws/) [90, 91], whereas GGDC 2.1 (http://ggdc.dsmz.de/distcalc2.php) using BLAST + alignment and recommended formula (2) were used to calculate the genome-to-genome distance [92, 93].
Additional in silico analyses of GLB 2draft-genome were performed using PathogenFinder 1.1 [94] and ResFinder 4.1 [95, 96] to estimate the number of pathogenicity determinants and antibiotic resistance genes (https://cge.cbs.dtu.dk/services).
3. Results
3.1. Antimicrobial and Enzymatic Potential Screening
Fourteen bacterial isolates were randomly selected (endophytes and from rhizosphere) and tested by overlay assay against S. aureus ATCC 29213, E. coli ATCC 11775, and C. albicans ATCC 10231 and phytopathogenic fungi as a primary selection on solid media. The results of the antimicrobial and enzymatic screening assay data are summarized in Tables 2 and 3. We observed that protease was the most abundant enzymatic activity detected, followed by pectinase at pH 8.0 (pectate lyase). Based on such results, we selected the isolate GLB2, which showed the antagonism activity against all tested pathogens and presented the enzymatic activity. We coined the isolate Burkholderia cepacia COPS strain in this study.
Table 2.
Results of the initial screening against pathogens.
| Pv110 | Pv168 | GLB10″ | GLB10′ | Roxo20 | Pv150 | Pv48 | Roxo19 | Roxo16 | Pv55 | WFRh72 | Pv46 | Unk3 | GLB2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human pathogens | ||||||||||||||
| Escherichia coli ATCC 25922 | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| Staphylococcus aureus ATCC 29213 | − | − | − | − | + | − | − | + | + | − | − | − | + | + |
| Candida albicans ATCC 10231 | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
|
| ||||||||||||||
| Phytopathogenic fungi | ||||||||||||||
| Moniliophthora perniciosa | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| Sclerotinia sclerotiorum | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium solani | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium verticillioides | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium proliferatum | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium oxysporum (bean) | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium oxysporum (cotton) | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Fusarium oxysporum (ATCC 2163) | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Phytophthora sojae | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| Ceratocystis paradoxa | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Colletotrichum sp. | − | − | − | − | − | − | − | − | − | + | + | − | + | + |
| Rhizopus microsporus | − | − | − | − | − | − | − | − | − | + | + | − | + | + |
| Alternaria alternata | − | − | − | − | − | − | − | − | − | + | − | − | + | + |
| Sphaceloma sp. (CNPUV 102) | + | − | − | − | − | − | − | − | − | + | − | − | + | + |
Table 3.
Enzymatic activity of bacteria isolated from Polygala paniculata.
| Amylase | Cellulase | Protease | Polygalacturonase | Pectate lyase | Lipase | Esterase | |
|---|---|---|---|---|---|---|---|
| PV 46 | + | − | + | + | + | + | − |
| PV 48 | − | − | − | − | + | − | − |
| PV 55 | − | − | + | − | − | + | + |
| PV 110 | − | − | − | − | + | − | − |
| PV 150 | + | + | + | + | + | − | − |
| PV 168 | − | − | + | − | − | − | − |
| WF.RH.72 | − | − | − | − | − | − | − |
| UNK 3 | − | − | + | − | − | − | − |
| GLB 2 | − | − | + | − | − | + | + |
| GLB 10′ | − | − | − | − | + | − | − |
| GLB 10″ | − | − | + | − | + | + | − |
| Roxo 16 | − | − | + | − | − | − | − |
| Roxo 19 | − | − | + | − | − | − | − |
| Roxo 20 | − | − | + | − | − | − | − |
3.2. Burkholderia cepacia COPS Genome
Root endophyte GLB2 isolate, which revealed a broad-spectrum and potent activity against all pathogens tested, was identified as Burkholderia cepacia based on 16s rRNA BLAST (GenBank accession number MN939546) search. The phylogenetic analysis revealed the isolate belongs to the Burkholderia sensu stricto group and is closely related to Bcc genomovar.
Genome sequencing of Burkholderia cepacia COPS strain generated 1,970,487 reads with an average length of 35–251 bp. The assembled genome estimated in 8.3 Mbp distributed in 80 contigs with an N50 of 275,353 bp. The functional annotation predicted 1885 genes, of which 1,838 are protein-coding genes (CDSs), 21 tRNAs, 26 misc RNAs, and 66.87% of a GC content. The draft-genome sequence was deposited in GenBank under accession number WIXR00000000.
Phylogenetic inferences (neighbor-joining and maximum likelihood) of COPS strain and Bcc reference strains of individual 16S rRNA and housekeeping genes atpD and phaC showed low-resolution bootstrap values. In addition, COPS strain is clustered separately from B. cepacia UCB 717 forming a single branch, whereas gltB, gyrB, lepA, recA, and trpB were strongly supported, as well lineages clearly grouped (Supplementary Material 1–8).
The multilocus sequence typing of Burkholderia cepacia COPS using the MLST-2.0 server revealed this strain belongs to the novel ST 1870 (ID 3851), with the following alleles numbers: atpD (235), gltB (707), trpB (21), recA (1), gyrB (1205), lepA (789), and phaC (607). Phylogenies regarding concatenated sequences (14833 bp) of the full length of seven housekeeping genes showed a significant branch support and strong association with B. cepacia UCB717 (Figure 2).
Figure 2.

Consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on the concatenated full length of seven housekeeping and 16S rDNA gene of B. cepacia COPS strain compared to reference sequences of Bcc members.
Pairwise digital DNA-DNA hybridization (dDDH) of COPS strain compared to 13 sequences of other Burkholderia cepacia strains revealed identity levels ranging from 87.90% to 80.70%, whereas ANIm, ANIb, and tetranucleotide frequency signature (TETRA) values of Burkholderia sp. LK4, B. reimsis BE51, and B. lata LK27 were interestingly slightly higher when compared to B. cepacia strains. Table 4 summarizes the genome level comparisons of COPS to other B. cepacia strains.
Table 4.
Genomic comparisons between Burkholderia cepacia COPS and B. cepacia strains based on genome comparisons and multilocus sequence analysis.
| Genome comparisons | Multilocus sequence analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Strain | GC (%) | ANIm (%) | ANIb (%) | dDDH (%) | Tetra | Locus | Identity (%) | Allele |
| Burkholderia cepacia RB-39 | 65.91 | 93.68 | 91.82 | 51.00 | 0.99738 | atpD | 100 | atpD 235 |
| Burkholderia cepacia DDS 7H-2 | 67.05 | 92.01 | 90.20 | 43.30 | 0.99559 | gltB | 100 | gltB 707 |
| Burkholderia cepacia DWS 16B-4 | 67.07 | 92.01 | 90.24 | 43.30 | 0.99561 | gyrB | 99.7797 | gyrB 1041 |
| Burkholderia cepacia LMG 16656 | 66.88 | 92.07 | 90.08 | 43.60 | 0.99553 | lepA | 99.4962 | lepA 3 |
| Burkholderia cepacia MSMB1302 | 66.81 | 96.22 | 95.19 | 67.00 | 0.99924 | phaC | 99.4805 | phaC 279 |
| Burkholderia cepacia LK13 | 66.47 | 98.72 | 98.04 | 87.80 | 0.99948 | recA | 100 | recA 1 |
| Burkholderia cepacia NBRC 14074 | 66.67 | 97.56 | 96.86 | 77.20 | 0.99929 | trpB | 100 | trpB 21 |
| Burkholderia cepacia NCTC10743 | 66.60 | 97.56 | 96.86 | 77.10 | 0.99932 | |||
| Burkholderia cepacia ATCC 25416 | 66.58 | 97.56 | 96.87 | 77.10 | 0.99935 | |||
| Burkholderia cepacia GG4 | 66.68 | 91.30 | 88.62 | 36.80 | 0.99608 | |||
| Burkholderia cepacia JBK9 | 66.82 | 92.35 | 90.59 | 45.00 | 0.99686 | |||
| Burkholderia cepacia MSMB591WGS | 66.43 | 98.71 | 98.13 | 87.40 | 0.99932 | |||
| Burkholderia cepacia MSMB1224WGS | 66.86 | 97.94 | 97.23 | 80.70 | 0.99892 | |||
| Burkholderia cepacia LO6 | 66.99 | 89.93 | 86.75 | 36.20 | 0.97175 | |||
| Burkholderia sp. LK4 | 66.46 | 98.78 | 98.05 | 87.80 | 0.99950 | |||
| Burkholderia reimsis BE51 | 66.37 | 98.72 | 98.05 | 86.50 | 0.99938 | |||
| Burkholderia lata LK27 | 66.76 | 98.53 | 96.91 | 87.90 | 0.99926 | |||
Bold values based on ANI, dDDH, and TETRA (threshold of ≥ 96%, ≥ 70%, and TETRA > 0.999%, respectively) represent that strains belong to the same genomic species, whereas in multilocus sequence analysis, bold values represent new alleles of housekeeping genes within Burkholderia spp.
Genome analysis performed using ResFinder 4.1 revealed the presence of ORFs corresponding to aph(3′)-IIa (99.75% identity) and blaTEM-116 (100% identity) genes, which confer resistance to aminoglycosides and beta-lactams, respectively. Nevertheless, B. cepacia COPS was estimated in 0.829 as a human pathogen, and its genome matched 53 pathogenic and five nonpathogenic families by PathogenFinder 1.1.
3.3. Antagonistic Effects of Burkholderia cepacia COPS Crude Extract
Regarding the bioactivity against bacterial and yeast pathogens in overlay assay, B. cepacia COPS potently inhibited S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922, M. luteus ATCC 9341, and C. albicans ATCC 10231. However, the NPE obtained from the COPS cultivation in ISP2 revealed inhibition against Gram-positive bacteria as well as A. baumannii ATCC 19606 and E. coli ATCC 25922 at 512 μg/mL. In addition, the NPE inhibited all phytopathogenic fungi in primary screening by a two-by-two streaking test. The inhibition index calculation was based on the inoculation of 500 μg/disk of B. cepacia COPS NPE. The phytopathogenic fungi M. perniciosa, S. sclerotirium, and C. paradoxa showed the highest inhibition percentages (89.32%, 85.53%, and 82.69%, respectively). The antagonism activity of NPE exceeded the positive control (74.17%) of M. perniciosa, whereas R. microsporus and F. oxysporum ATCC 2163 were less inhibited (6.59% and 7.17%, respectively) (Figure 3 and Table 5).
Figure 3.

Antimicrobial activity of NPE (a) produced by B. cepacia COPS. Columns (b) and (c) correspond to different crude extracts' antibacterial activity compared to overlay assay (top to bottom: Bacillus cereus, Escherichia coli, and Enterococcus faecalis). Lanes (d) COPS NPE, (e) benomyl, as positive control, and (f) negative control represent the antifungal effect. From top to bottom: Moniliophthora perniciosa, Ceratocystis paradoxa, Sclerotinia sclerotiorum, Alternaria alternata, and Fusarium verticillioides.
Table 5.
Antagonistic activity of B. cepacia strain COPS NPE toward phytopathogenic fungi and pathogenic bacteria.
| Phytopathogenic fungi | Public health pathogens | ||||
|---|---|---|---|---|---|
| NPE treatment | Benomyl | GLB2 treatment | |||
| AI (%) | AI (%) | MIC (μg/mL) | IC50 (μg/mL) | ||
| Moniliophthora perniciosa | 89.32 | 74.17 | Staphylococcus aureus ATCC 25923 | 128 | 58.36 |
| Sclerotinia sclerotiorum | 85.53 | 86.26 | Staphylococcus epidermidis ATCC 35984 | 32 | 22.49 |
| Fusarium solani | 37.97 | 85.54 | Enterococcus faecium ATCC 700221 | 32 | 24.11 |
| Fusarium verticillioides | 27.10 | 92.79 | Enterococcus faecalis ATCC 29212 | 64 | 26.34 |
| Fusarium proliferatum | 26.27 | 92.17 | Acinetobacter baumannii ATCC 19606 | 512 | 298.2 |
| Fusarium oxysporum (bean) | 15.95 | 90.58 | Escherichia coli ATCC 25922 | 512 | 103.4 |
| Fusarium oxysporum (cotton) | 13.21 | 89.79 | Klebsiella pneumoniae ATCC 700603 | >512∗ | N.D. |
| Fusarium oxysporum (ATCC 2163) | 7.17 | 94.42 | Pseudomonas aeruginosa ATCC 14502 | >512∗∗ | N.D. |
| Phytophthora sojae | 61.15 | 100.00 | |||
| Ceratocystis paradoxa | 82.69 | 100.00 | |||
| Colletotrichum sp. | 42.27 | 100.00 | |||
| Rhizopus microsporus | 6.59 | 26.53 | |||
| Alternaria alternata | 22.53 | 87.48 | |||
| Sphaceloma sp. (CNPUV 102) | 61.45 | 27.52 | |||
∗Partial inhibition at 512 μg/mL (14.09%); ∗∗partial inhibition at 512 μg/mL (25.36%).
3.4. Activity against Leishmania spp
We tested the activity of B. cepacia COPS NPE in vitro against Leishmania infantum and Leishmania major. A 24-hour treatment with NPE concentrations ranging from 200 to 1 μg/mL potentially inhibited L. infantum promastigotes with cell viability ranging from 27% to 78.25%, respectively, with IC50 of 86.6 μg/mL. Moreover, the NPE in the highest concentration tested was more effective than amphotericin B at 100 μM (28.6% cell viability).
On the other hand, the effect of COPS NPE against Leishmania major was not so potent compared to L. infantum. The results show 57.30% cell viability in a 200 μg/mL concentration and an IC50 of 94.55 μg/mL. Figure 4 summarizes the enzymatic and antileishmanial effects of B. cepacia COPS.
Figure 4.

Evaluation of enzymatic production of Burkholderia cepacia COPS in solid media: (a) protease; (b) esterase; (c) lipase. Activity of B. cepacia COPS NPE at different concentrations against promastigotes of L. infantum (d) and L. major (e) after 24 hours of exposure. Values represent two independent experiments performed in triplicate. ∗∗∗Indicates statistical significance compared to the negative control, with values of p < 0.001.
4. Discussion
Burkholderia spp. are nonfermenting bacteria spread worldwide and highly able to adapt to various ecological niches [43, 46, 97–99]. Although Bcc is considered potentially life-threatening as an opportunistic pathogen in hospital environments, especially in patients with cystic fibrosis, Burkholderia spp. are versatile for biotechnological applications, and their potential has been exploited for bioremediation, plants growth promotion, biological control, and broad-spectrum agents in several members of this group [41, 46, 47, 100].
Burkholderia genus (sensu lato) comprises more than 100 species that possess the ability to adapt to various ecological niches [99, 101]. Members of this complex group exhibit genomes ranging from 7 to 9 Mbp, resulting in several taxonomic rearrangements [46, 99], rapid mutation, and adaptation [102]. Bcc species exhibit high genetic similarity and is phenotypically indistinguishable [102–104], leading to often misidentifications interfering the effective treatment and epidemiological studies [52, 105].
Our analyses concerning COPS strain for pathogenicity determinants using PathogenFinder 1.1 revealed a prediction of pathogenic potential of 0.829, whereas the reference B. cepacia ATCC 25416 was 0.774. Interestingly, Z-scores of TETRA correlation were slightly higher (Burkholderia sp. LK4, B. reimsis BE51, and B. lata LK27) when compared to B. cepacia strains.
The analysis of sequence variations of recA and hisA genes offers further discriminatory support at species-level identification [53, 104, 106]. However, multilocus sequence typing offers more sensitivity to identify species within Bcc [57, 107–109]. Our results showed a significant branch support (100%) and a strong association with B. cepacia UCB717 and revealed novel alleles of gyrB, lepA, and phaC, which led us to describe the novel ST 1870. As mentioned, Burkholderia spp. may be found in a wide range of niches, and genetic variations might occur in response to niche adaptation [41, 109].
Regarding the individual alignments of atpD and phaC, our results showed low-resolution bootstrap values, which corroborate inconsistencies found in MLST, ANI, and DNA hybridization of the soil isolate Burkholderia catarinensis sp. nov., formerly reported as B. cepacia [110]. Analysis by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) enabled the proper identification of novel species of Burkholderiales. Interestingly, B. catarinensis exhibits physiological characteristics that differ from most other Bcc species [52].
In summary, the present study confirms that COPS strain is closely related to B. cepacia based on the whole-genome ANI, individual housekeeping gene sequence analysis, and MLST. Moreover, we present for the first time the identification of a B. cepacia symbiotically/endophytically associated with Polygala paniculata suggesting that Polygala genus plays a role in harboring microorganisms for biotechnological applications.
The detection of in vitro production of active compounds may be tricky. Usually, quorum sensing (QS) also controls the production of multiple antimicrobial substances. The cell density can promote a signaling system that transcribes certain genes for their interaction with their hosts and increases resistance to stresses [43, 111]. However, the presence of nutrients in the fermentation medium might affect the biosynthesis of QS signaling molecules, such as N-acyl homoserine lactones. Keum et al. [112] demonstrated that glucose (present in ISP2 at 4 g/L in its composition) increases the biomass, but suppresses the production of pyrrolnitrin, which is effective against fungi, yeasts, and Gram-positive bacteria [113]. Nevertheless, Figure 4 shows that the COPS NPE produced by the cultivation in ISP2 still exhibited potent inhibition against phytopathogenic fungi (lanes D, E, and F). Interestingly, regarding cell proliferation, the cultivation of B. cepacia COPS in ISP2 showed higher cell density comparing to other media, such as Luria–Bertani medium [114], PDB [67], and 2S4G [115]. However, in disk diffusion assay, the NPEs obtained by cultivation in different media presented a nonsignificant activity (lane B). In contrast, the overlay assay using ISP2 showed a moderate activity against B. cereus and higher inhibition zones against E. coli and E. faecalis (lane C). Thus, these data corroborate related studies [112, 116, 117] concerning multiple secondary metabolites' production.
In agrobiology, the broad-spectrum activity of microorganisms that inhibit plant pathogens is critically important for biological control because the ability to antagonize phytopathogens can indirectly promote the host plant growth [99, 118]. In this context, Orlandelli et al. [118] evaluated antagonism and competitive interactions of endophytic fungi isolated from Piper hispidum against Alternaria alternata, Colletotrichum sp., Phyllosticta citricarpa, and Moniliophthora perniciosa. Although fungus Lasiodiplodia theobromae showed activity against all tested phytopathogens in a dual culture assay, it exhibited antagonism indexes of 60.09% against M. perniciosa, 64.79% in A. alternata, and 54.16% in Colletotrichum sp. In our study, the NPE of Burkholderia cepacia COPS showed better inhibition toward M. perniciosa, thus exceeding the positive control (74.17%). On the other hand, the COPS crude extract showed mild effects toward A. alternata (22.53%) and Colletotrichum sp. (42.27%). The phytopathogenic fungus Moniliophthora perniciosa, which causes the witches' broom disease in cacao crops, is responsible for 90% losses in the cacao annual production [119, 120]. Therefore, it is of interest to have a microorganism, such as B. cepacia COPS, that could help in the cacao crops infection control.
Interestingly, de Almeida Lopes et al. [121] isolated three endophytic strains of Bcc from soybean plants. The cultivation in nutrient broth produced bioactive lipopeptides, extracted by different methods (methanol, ethyl acetate, and ammonium sulfate precipitation), and qualitatively tested regarding their capacity to inhibit fungal (S. sclerotiorum, P. sojae, and R. solani) and bacterial (X. axonopodis pv. glycines and P. savastanoi pv. glycinea) plant pathogens. The inhibition rates against the phytopathogenic fungi exceeded 70%; whereas, in our study, 200 μg of the COPS NPE promoted a potent inhibition of Sclerotinia sclerotiorum and showed a moderate activity against P. sojae.
Our analysis revealed that COPS NPE can potentially inhibit C. paradoxa but exhibits moderate and slight activities against Colletotrichum sp. and Fusarium verticillioides, respectively. Such phytopathogenic fungi and others are responsible for high losses in the production of several crops worldwide [122, 123]. C. paradoxa causes the black rot postharvest disease in pineapple [124] and also infects sugarcane [125, 126], palm trees, cacao plants, and several other crops [127]. F. verticillioides is a producer of fumonisin, a carcinogenic mycotoxin [128], and other species have been described as emergent and opportunistic pathogens in humans [129]. Fávaro et al. [125] monitored E. nigrum endophytically inoculated in sugarcane plants, and its extract significantly reduced the diameter of Fusarium verticillioides, Colletotrichum falcatum, Ceratocystis paradoxa, and Xanthomonas albilineans colonies at concentrations ranging from 0.1 to 2.0 mg/mL.
As discussed above, the COPS strain was capable of producing lipase in solid medium, and its NPE potentially inhibited L. infantum and L. major. These observations corroborate the work of Alves et al. [130] that investigated the antileishmanial activity of crude extracts of lipase-producing endophytic fungi toward Leishmania amazonensis. The antileishmanial activity of lipases of Vermisporium sp. (78.88%), Emericella nidulans (39.65%), Dichotomophtora portulacae (63.17%), and Dichotomophtora boerhaaviae (98.13%) was detected at 5 mg/mL in amastigote forms, suggesting an enhancement of antileishmanial activity by lipases due to their thermal stability and resistance to several organic solvents, including alcohols [131]. Therefore, a detailed analysis of compounds produced by B. cepacia COPS is fundamental for a complete understanding of its potent antileishmanial effect.
Considering that the leishmaniasis treatment with pentavalent antimonials is known to be ineffective and unsafe and therapies based on pentamidine and amphotericin B are considered toxic and exhibit recurrence rates, it is clear that new treatment alternatives are necessary [132–136]. Although widely described as a producer of antibacterial and antifungal compounds [41, 42, 137–139], we emphasize that the Burkholderia species' antileishmanial activity was unknown so far. As we demonstrated in this study, Burkholderiales may offer promising candidates to treat neglected diseases of which resistance and toxicity of current treatments represent a global public health concern.
5. Conclusions
This research's novelty lies in the isolation of rhizospheric and endophytic bacteria associated with Polygala paniculata and isolation and biological activity determination of the root endophyte Burkholderia cepacia COPS strain. Our results demonstrated that Polygala paniculata is a promising source of microorganisms for the fight against Leishmania spp., bacteria of clinical importance, and phytopathogens.
Acknowledgments
This study was financially supported by National Council for Scientific and Technological Development (CNPq; 140125/2015-9) and São Paulo State Research Support Foundation (FAPESP) through CEPID Program—CIBFar Project (2013/07600-3 and 2019/04788-8) and the regular project (2016/13423-5).
Abbreviations
- AI:
Antagonism index
- ANIb:
Average nucleotide identity based on BLAST
- ANIm:
Average nucleotide identity based on MUMmer
- Bcc:
Burkholderia cepacia complex
- BHI:
Brain-heart infusion agar
- CFU:
Colony forming units
- CLSI:
Clinical and Laboratory Standards Institute
- dDDH:
Digital DNA-DNA hybridization
- DMSO:
Dimethyl sulfoxide
- ISP2:
International Streptomyces Project medium 2
- MALDI-TOF MS:
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MIC:
Minimum inhibitory concentration
- MHCA:
Müeller–Hinton cation adjusted
- MLSA:
Multilocus sequence analysis
- MLST:
Multilocus sequence typing
- NPE:
Natural products extract
- OD:
Optical density
- PBS:
Phosphate-buffered solution
- PDA:
Potato dextrose agar
- QS:
Quorum sensing
- ST:
Sequence type
- TETRA:
Tetranucleotide frequency
- TSA:
Tryptic soy agar
- TSB:
Tryptic soy broth.
Data Availability
The data used to support the findings of this study are found in laboratory notebooks of Laboratory of Microbiology and Biomolecules (LaMiB), Department of Morphology and Pathology, Federal University of São Carlos, Brazil.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Felipe de Paula Nogueira Cruz, Paulo Teixeira Lacava, Fernanda de Freitas Aníbal, and Cristina Paiva de Sousa contributed to conception and designed the study. Leonardo Maurici Borges identified the plant. Ilana L B C Camargo and Felipe de Paula Nogueira Cruz contributed to genome sequencing. Felipe de Paula Nogueira Cruz and Cristina Paiva de Sousa analyzed and interpreted data. Felipe de Paula Nogueira Cruz, Ailton Ferreira de Paula, Camila Tita Nogueira, and Paulo Henrique Marques de Andrade performed experiments. Felipe de Paula Nogueira Cruz, Paulo Teixeira Lacava, Ilana L B C Camargo, Leonardo Maurici Borges, Fernanda de Freitas Aníbal, and Cristina Paiva de Sousa approved the final version to be submitted.
Supplementary Materials
Supplementary Figure 1: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length 16S rDNA gene of B. cepacia COPS strain compared to reference sequences of Bcc members. Supplementary Figure 2: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length atpD gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 3: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length gltB gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 4: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length gyrB gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 5: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length lepA gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 6: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length phaC gene of B. cepacia COPS strain compared to reference sequences of Bcc members. Supplementary Figure 7: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length recA gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 8: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length trpB gene of B. cepacia COPS strain compared to reference sequences of Bcc.
References
- 1.Giang P. M., Otsuka H. New compounds and potential candidates for drug discovery from medicinal plants of Vietnam. Chemical and Pharmaceutical Bulletin. 2018;66(5):493–505. doi: 10.1248/cpb.c17-00628. [DOI] [PubMed] [Google Scholar]
- 2.Gómez O. C., Luiz J. H. H. Endophytic fungi isolated from medicinal plants: future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Applied Microbiology and Biotechnology. 2018;102(21):9105–9119. doi: 10.1007/s00253-018-9344-3. [DOI] [PubMed] [Google Scholar]
- 3.Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):p. 258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Klimova E., Rodríguez-Peña K., Sánchez S. Endophytes as sources of antibiotics. Biochemical Pharmacology. 2017;134:1–17. doi: 10.1016/j.bcp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Ancheeva E., Daletos G., Proksch P. Bioactive secondary metabolites from endophytic fungi. Current Medicinal Chemistry. 2020;27(11):1836–1854. doi: 10.2174/0929867326666190916144709. [DOI] [PubMed] [Google Scholar]
- 6.Elias L. M., Fortkamp D., Sartori S. B., et al. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Brazilian Journal of Microbiology. 2018;49(4):840–847. doi: 10.1016/j.bjm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo J. L., Maccheroni W., Jr., Pereira J. O., De Araújo W. L. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electronic Journal of Biotechnology. 2000;3:15–16. doi: 10.2225/vol3-issue1-fulltext-4. [DOI] [Google Scholar]
- 8.Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic Microorganisms. Journal of Natural Products. 2004;67(2):257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 9.Pacifico D., Squartini A., Crucitti D., et al. The role of the endophytic microbiome in the grapevine response to environmental triggers. Frontiers in Plant Science. 2019;10:p. 1256. doi: 10.3389/fpls.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira F. L. P., Fernandes S. B. O., Reis G. M., et al. Atividade analgésica e antiedematogênica de Polygala paniculata L. (Polygalaceae) selvagem e obtida por micropropagação. Revista Brasileira de Farmacognosia. 2005;15(4):310–315. doi: 10.1590/S0102-695X2005000400009. [DOI] [Google Scholar]
- 11.Bettio L. E. B., Machado D. G., Cunha M. P., et al. Antidepressant-like effect of extract from Polygala paniculata: involvement of the monoaminergic systems. Pharmaceutical Biology. 2011;49(12):1277–1285. doi: 10.3109/13880209.2011.621958. [DOI] [PubMed] [Google Scholar]
- 12.Frescura V. D., Laughinghouse H. D., IV do Canto-Dorow T. S., Tedesco S. B. Pollen viability of Polygala paniculata L. (Polygalaceae) using different staining methods. Biocell: Official Journal of the Sociedades Latinoamericanas de Microscopia Electronica. 2012;36(3):143–145. [PubMed] [Google Scholar]
- 13.Lacaille-Dubois M.-A., Delaude C., Mitaine-Offer A.-C. A review on the phytopharmacological studies of the genus Polygala. Journal of Ethnopharmacology. 2020;249 doi: 10.1016/j.jep.2019.112417.112417 [DOI] [PubMed] [Google Scholar]
- 14.Jin B. Y., Park J. Studies on the alkaloidal components of Polygala tenuifolia willd. Zhongguo Zhong Yao Za Zhi. 1993;18(11):675–677. [PubMed] [Google Scholar]
- 15.Dall’Acqua S., Innocenti G., Viola G., Piovan A., Caniato R., Cappelletti E. M. Cytotoxic compounds from Polygala vulgaris. Chemical & Pharmaceutical Bulletin. 2002;50(11):1499–1501. doi: 10.1248/cpb.50.1499. [DOI] [PubMed] [Google Scholar]
- 16.Dall’Acqua S., Viola G., Cappelletti E. M., Innocenti G. Xanthones from Polygala alpestris (Rchb.) Zeitschrift für Naturforschung C: A Journal of Biosciences. 2004;59(5-6):335–338. doi: 10.1515/znc-2004-5-608. [DOI] [PubMed] [Google Scholar]
- 17.Nagai T., Suzuki Y., Kiyohara H., et al. Onjisaponins, from the root of Polygala tenuifolia Willdenow, as effective adjuvants for nasal influenza and diphtheria-pertussis-tetanus vaccines. Vaccine. 2001;19(32):4824–4834. doi: 10.1016/s0264-410x(01)00215-8. [DOI] [PubMed] [Google Scholar]
- 18.Jia H., Jiang Y., Ruan Y., et al. Tenuigenin treatment decreases secretion of the Alzheimer’s disease amyloid β-protein in cultured cells. Neuroscience Letters. 2004;367(1):123–128. doi: 10.1016/j.neulet.2004.05.093. [DOI] [PubMed] [Google Scholar]
- 19.Hamburger M., Gupta M., Hostettmann K. Coumarins from Polygala paniculata. Planta Medica. 1985;51(3):215–217. doi: 10.1055/s-2007-969460. [DOI] [PubMed] [Google Scholar]
- 20.Cheong M.-H., Lee S.-R., Yoo H.-S., et al. Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-κB activation in lipopolysaccharide-induced BV2 microglial cells. Journal of Ethnopharmacology. 2011;137(3):1402–1408. doi: 10.1016/j.jep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Arruda-Silva F., Nascimento M. V. P. S., Luz A. B. G., et al. Polygala molluginifolia A. St.-Hil. and Moq. prevent inflammation in the mouse pleurisy model by inhibiting NF-κB activation. International Immunopharmacology. 2014;19(2):334–341. doi: 10.1016/j.intimp.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Cao Q., Jiang Y., Cui S.-Y., et al. Tenuifolin, a saponin derived from Radix Polygalae, exhibits sleep-enhancing effects in mice. Phytomedicine. 2016;23(14):1797–1805. doi: 10.1016/j.phymed.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Pizzolatti M. G., Koga A. H., Grisard E. C., Steindel M. Trypanocidal activity of extracts from Brazilian Atlantic rain forest plant species. Phytomedicine. 2003;10(5):422–426. doi: 10.1078/0944-7113-00252. [DOI] [PubMed] [Google Scholar]
- 24.Nucci-Martins C., Nascimento L. F., Venzke D., et al. Antinociceptive effect of hydroalcoholic extract and isoflavone isolated from Polygala molluginifolia in mice: evidence for the involvement of opioid receptors and TRPV1 and TRPA1 channels. Phytomedicine. 2016;23(5):429–440. doi: 10.1016/j.phymed.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Guo C., Shen J., Meng Z., Yang X., Li F. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine. 2016;23(2):149–155. doi: 10.1016/j.phymed.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Huang X. B., Chen Y. J., Chen W. Q., Wang N. Q., Wu X. L., Liu Y. Neuroprotective effects of tenuigenin on neurobehavior, oxidative stress, and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Brain Circulation. 2018;4(1):24–32. doi: 10.4103/bc.bc_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S., Sun Y., Han Z., Bu X., Yu W., Wang J. Retracted: cytoprotective effects of euxanthone against ox-LDL-induced endothelial cell injury is mediated via Nrf2. Life Sciences. 2019;223:174–184. doi: 10.1016/j.lfs.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L., Liu X., Li D., Sun S., Wang Y., Sun X. Retracted: autophagy is a pro-survival mechanism in ovarian cancer against the apoptotic effects of euxanthone. Biomedicine & Pharmacotherapy. 2018;103:708–718. doi: 10.1016/j.biopha.2018.04.090. [DOI] [PubMed] [Google Scholar]
- 29.Zou J., Wang Y., Liu M., et al. Retracted: euxanthone inhibits glycolysis and triggers mitochondria‐mediated apoptosis by targeting hexokinase 2 in epithelial ovarian cancer. Cell Biochemistry and Function. 2018;36(6):303–311. doi: 10.1002/cbf.3349. [DOI] [PubMed] [Google Scholar]
- 30.Johann S., Mendes B. G., Missau F. C., Resende M. A. d., Pizzolatti M. G. Antifungal activity of five species of Polygala. Brazilian Journal of Microbiology. 2011;42(3):1065–1075. doi: 10.1590/s1517-83822011000300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caraballo-Rodríguez A. M., Dorrestein P. C., Pupo M. T. Molecular inter-kingdom interactions of endophytes isolated from Lychnophora ericoides. Scientific Reports. 2017;7(1):p. 5373. doi: 10.1038/s41598-017-05532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigliotta G., Giordano D., Verdino A., et al. New compounds for a good old class: synthesis of two Β-lactam bearing cephalosporins and their evaluation with a multidisciplinary approach. Bioorganic & Medicinal Chemistry. 2020;28(4) doi: 10.1016/j.bmc.2019.115302.115302 [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou K. C., Rigol S. A brief history of antibiotics and select advances in their synthesis. The Journal of Antibiotics. 2018;71(2):153–184. doi: 10.1038/ja.2017.62. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto A., Takahashi Y. Endophytic actinomycetes: promising source of novel bioactive compounds. The Journal of Antibiotics. 2017;70(5):514–519. doi: 10.1038/ja.2017.20. [DOI] [PubMed] [Google Scholar]
- 35.Brauer V. S., Rezende C. P., Pessoni A. M., et al. Antifungal agents in agriculture: friends and foes of public health. Biomolecules. 2019;9(10):p. 521. doi: 10.3390/biom9100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullen M. G., Thompson L. J., Carolan J. C., Stout J. C., Stanley D. A. Fungicides, herbicides and bees: a systematic review of existing research and methods. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0225743.e0225743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omomowo O. I., Babalola O. O. Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms. 2019;7(11):p. 481. doi: 10.3390/microorganisms7110481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcântara L. M., Ferreira T. C. S., Gadelha F. R., Miguel D. C. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. International Journal for Parasitology: Drugs and Drug Resistance. 2018;8(3):430–439. doi: 10.1016/j.ijpddr.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bekhit A. A., El-Agroudy E., Helmy A., Ibrahim T. M., Shavandi A., Bekhit A. E.-D. A. Leishmania treatment and prevention: natural and synthesized drugs. European Journal of Medicinal Chemistry. 2018;160:229–244. doi: 10.1016/j.ejmech.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Cassini A., Högberg L. D., Plachouras D., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. The Lancet Infectious Diseases. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunakom S., Eustáquio A. S. Burkholderiaas a source of natural products. Journal of Natural Products. 2019;82(7):2018–2037. doi: 10.1021/acs.jnatprod.8b01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S.-E., Novak J., Austin F. W., et al. Occidiofungin, a unique antifungal glycopeptide produced by a strain of Burkholderia contaminans. Biochemistry. 2009;48(35):8312–8321. doi: 10.1021/bi900814c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Depoorter E., Bull M. J., Peeters C., Coenye T., Vandamme P., Mahenthiralingam E. Burkholderia: an update on taxonomy and biotechnological potential as antibiotic producers. Applied Microbiology and Biotechnology. 2016;100(12):5215–5229. doi: 10.1007/s00253-016-7520-x. [DOI] [PubMed] [Google Scholar]
- 44.Song L., Jenner M., Masschelein J., et al. Discovery and biosynthesis of gladiolin: a Burkholderia gladioli antibiotic with promising activity against Mycobacterium tuberculosis. Journal of the American Chemical Society. 2017;139(23):7974–7981. doi: 10.1021/jacs.7b03382. [DOI] [PubMed] [Google Scholar]
- 45.Esmaeel Q., Miotto L., Rondeau M., et al. Paraburkholderia phytofirmans PsJN-plants interaction: from perception to the induced mechanisms. Frontiers in Microbiology. 2018;9:p. 2093. doi: 10.3389/fmicb.2018.02093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furlan J. P. R., Pitondo-Silva A., Braz V. S., Gallo I. F. L., Stehling E. G. Evaluation of different molecular and phenotypic methods for identification of environmental Burkholderia cepacia complex. World Journal of Microbiology and Biotechnology. 2019;35(3):p. 39. doi: 10.1007/s11274-019-2614-0. [DOI] [PubMed] [Google Scholar]
- 47.Mullins A. J., Murray J. A. H., Bull M. J., et al. Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nature Microbiology. 2019;4(6):996–1005. doi: 10.1038/s41564-019-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunakom S., Eustáquio A. S. Heterologous production of lasso peptide Capistruin in a Burkholderia host. ACS Synthetic Biology. 2020;9(2):241–248. doi: 10.1021/acssynbio.9b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coenye T., Vandamme P., Govan J. R. W., LiPuma J. J. Taxonomy and identification of the Burkholderia cepacia complex. Journal of Clinical Microbiology. 2001;39(10):3427–3436. doi: 10.1128/JCM.39.10.3427-3436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janda J. M., Abbott S. L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. Journal of Clinical Microbiology. 2007;45(9):2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinucci M., Roscetto E., Iula V. D., Votsi A., Catania M. R., De Gregorio E. Accurate identification of members of the Burkholderia cepacia complex in cystic fibrosis sputum. Letters in Applied Microbiology. 2016;62(3):221–229. doi: 10.1111/lam.12537. [DOI] [PubMed] [Google Scholar]
- 52.Bach E., Sant’Anna F. H., Magrich Dos Passos J. F., et al. Detection of misidentifications of species from the Burkholderia cepacia complex and description of a new member, the soil bacterium Burkholderia catarinensis sp. nov. Pathogens and Disease. 2017;75(6):p. 75. doi: 10.1093/femspd/ftx076. [DOI] [PubMed] [Google Scholar]
- 53.Martina P., Leguizamon M., Prieto C. I., et al. Burkholderia puraquae sp. nov., a novel species of the Burkholderia cepacia complex isolated from hospital settings and agricultural soils. International Journal of Systematic and Evolutionary Microbiology. 2018;68(1):14–20. doi: 10.1099/ijsem.0.002293. [DOI] [PubMed] [Google Scholar]
- 54.Godoy D., Randle G., Simpson A. J., et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. Journal of Clinical Microbiology. 2003;41(5):2068–2079. doi: 10.1128/jcm.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spilker T., Baldwin A., Bumford A., Dowson C. G., Mahenthiralingam E., LiPuma J. J. Expanded multilocus sequence typing for Burkholderia species. Journal of Clinical Microbiology. 2009;47(8):2607–2610. doi: 10.1128/JCM.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estrada-de los Santos P., Vinuesa P., Martínez-Aguilar L., Hirsch A. M., Caballero-Mellado J. Phylogenetic analysis of Burkholderia species by multilocus sequence analysis. Current Microbiology. 2013;67(1):51–60. doi: 10.1007/s00284-013-0330-9. [DOI] [PubMed] [Google Scholar]
- 57.Gautam V., Patil P. P., Kumar S., et al. Multilocus sequence analysis reveals high genetic diversity in clinical isolates of Burkholderia cepacia complex from India. Scientific Reports. 2016;6(1) doi: 10.1038/srep35769.35769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price E. P., MacHunter B., Spratt B. G., Wagner D. M., Currie B. J., Sarovich D. S. Improved multilocus sequence typing of Burkholderia pseudomallei and closely related species. Journal of Medical Microbiology. 2016;65(9):992–997. doi: 10.1099/jmm.0.000312. [DOI] [PubMed] [Google Scholar]
- 59.Birnie E., van’t Hof S., Bijnsdorp A., et al. Identification of Burkholderia thailandensis with novel genotypes in the soil of central Sierra Leone. PLoS Neglected Tropical Diseases. 2019;13(6) doi: 10.1371/journal.pntd.0007402.e0007402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X., Zheng X., Huang M., Liu L. A comparative genomic analysis of small-colony variant and wild-type Burkholderia pseudomallei in a patient with bacterial liver abscess. Journal of Global Antimicrobial Resistance. 2020;21:16–21. doi: 10.1016/j.jgar.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Zhu X., Chen H., Li S., et al. Molecular characteristics of Burkholderia pseudomallei collected from humans in Hainan, China. Frontiers in Microbiology. 2020;11:p. 778. doi: 10.3389/fmicb.2020.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linnaeus C. Systema Naturae. (10th) 1759;2:p. 1154. [Google Scholar]
- 63.Marques M. C. M., Gomes K. Polygalaceae in Wanderley. In: Wanderley M. G. L., Shepherd G. J., Giulietti A. M., editors. Flora Fanerogâmica do Estado de São Paulo. Vol. 2. São Paulo, Brazil: Instituto deBotânica; 2002. pp. 229–259. [Google Scholar]
- 64.Araújo W. L., Quecine M. C., Lacava P. T., et al. Microrganismos Endofíticos: Aspectos Teóricos e Práticos de Isolamento e Caracterização. Santarém, Brazil: UFOPA; 2014. [Google Scholar]
- 65.Bogas A. C., Ferreira A. J., Araújo W. L., et al. Endophytic bacterial diversity in the phyllosphere of Amazon Paullinia cupana associated with asymptomatic and symptomatic anthracnose. Springerplus. 2015;4(1):p. 258. doi: 10.1186/s40064-015-1037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andreote F. D., Mendes R., Dini-Andreote F., et al. Transgenic tobacco revealing altered bacterial diversity in the rhizosphere during early plant development. Antonie Van Leeuwenhoek. 2008;93(4):415–424. doi: 10.1007/s10482-007-9219-6. [DOI] [PubMed] [Google Scholar]
- 67.Rojas-Rojas F. U., Salazar-Gómez A., Vargas-Díaz M. E., et al. Broad-spectrum antimicrobial activity by Burkholderia cenocepacia TAtl-371, a strain isolated from the tomato rhizosphere. Microbiology. 2018;164(9):1072–1086. doi: 10.1099/mic.0.000675. [DOI] [PubMed] [Google Scholar]
- 68.Kurnianto M. A., Kusumaningrum H. D., Lioe H. N. Characterization of Streptomyces isolates associated with estuarine fish Chanos chanos and profiling of their antibacterial metabolites-crude-extract. International Journal of Microbiology. 2020;2020:12. doi: 10.1155/2020/8851947.8851947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shirling E. B., Gottlieb D. Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology. 1966;16(3):313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- 70.Quiroga E. N., Sampietro A. R., Vattuone M. A. Screening antifungal activities of selected medicinal plants. Journal of Ethnopharmacology. 2001;74(1):89–96. doi: 10.1016/s0378-8741(00)00350-0. [DOI] [PubMed] [Google Scholar]
- 71.Silva M. C. S. e., Polonio J. C., Quecine M. C., et al. Endophytic cultivable bacterial community obtained from the Paullinia cupana seed in Amazonas and Bahia regions and its antagonistic effects against Colletotrichum gloeosporioides. Microbial Pathogenesis. 2016;98:16–22. doi: 10.1016/j.micpath.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Specian V., Costa A. T., Felber A. C., Polonio J. C., Azevedo J. L., Pamphile J. A. Molecular phylogeny and biotechnological potential of bacterial endophytes associated with Malpighia emarginata. Genetics and Molecular Research. 2016;15(2):p. 15. doi: 10.4238/gmr.15027777. [DOI] [PubMed] [Google Scholar]
- 73.Park S. R., Tripathi A., Wu J., et al. Discovery of cahuitamycins as biofilm inhibitors derived from a convergent biosynthetic pathway. Nature Communications. 2016;7(1) doi: 10.1038/ncomms10710.10710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. 28th. Wayne, PA, USA: Clinical and Laboratory Standard Institute; 2018. [Google Scholar]
- 75.Okonechnikov K., Golosova O., Fursov M. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 76.Bolger A. M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bankevich A., Nurk S., Antipov D., et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 80.Aziz R. K., Bartels D., Best A. A., et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9(1):p. 75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galardini M., Biondi E. G., Bazzicalupo M., Mengoni A. CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code for Biology and Medicine. 2011;6(1):p. 11. doi: 10.1186/1751-0473-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lagesen K., Hallin P. F., Rødland E., Stærfeldt H. H., Rognes T., Ussery D. W. RNAmmer: Consistent annotation of rRNA genes in genomic sequences. Nucleic Acids Research. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mega X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and evolution. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 85.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences. 2004;101(30):11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurtz S., Phillippy A., Delcher A. L., et al. Versatile and open software for comparing large genomes. Genome Biology. 2004;5(2):p. R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goris J., Konstantinidis K. T., Klappenbach J. A., Coenye T., Vandamme P., Tiedje J. M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. International Journal of Systematic and Evolutionary Microbiology. 2007;57(1):81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 88.Camacho C., Coulouris G., Avagyan V., et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):p. 421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teeling H., Meyerdierks A., Bauer M., Amann R., Glöckner F. O. Application of tetranucleotide frequencies for the assignment of genomic fragments. Environmental Microbiology. 2004;6(9):938–947. doi: 10.1111/j.1462-2920.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 90.Richter M., Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proceedings of the National Academy of Sciences. 2009;106(45):19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richter M., Rosselló-Móra R., Oliver Glöckner F., Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32(6):929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meier-Kolthoff J. P., Auch A. F., Klenk H.-P., Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14(1):p. 60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meier-Kolthoff J. P., Klenk H.-P., Göker M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. International Journal of Systematic and Evolutionary Microbiology. 2014;64(2):352–356. doi: 10.1099/ijs.0.056994-0. [DOI] [PubMed] [Google Scholar]
- 94.Cosentino S., Voldby Larsen M., Møller Aarestrup F., Lund O. PathogenFinder—distinguishing friend from foe using bacterial whole genome sequence data. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077302.e77302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zankari E., Hasman H., Cosentino S., et al. Identification of acquired antimicrobial resistance genes. Journal of Antimicrobial Chemotherapy. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bortolaia V., Kaas R. S., Ruppe E., et al. ResFinder 4.0 for predictions of phenotypes from genotypes. Journal of Antimicrobial Chemotherapy. 2020;75(12):p. 3491. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paungfoo-Lonhienne C., Lonhienne T. G. A., Yeoh Y. K., et al. Crosstalk between sugarcane and a plant-growth promoting Burkholderia species. Scientific Reports. 2016;6(1):p. 37389. doi: 10.1038/srep37389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen T. T., Lee H.-H., Park J., Park I., Seo Y.-S. Computational identification and comparative analysis of secreted and transmembrane proteins in six Burkholderia species. The Plant Pathology Journal. 2017;33(2):148–162. doi: 10.5423/PPJ.OA.11.2016.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mannaa M., Park I., Seo Y.-S. Genomic features and insights into the taxonomy, virulence, and benevolence of plant-associated Burkholderia species. International Journal of Molecular Sciences. 2018;20(1):p. 121. doi: 10.3390/ijms20010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendes R., Pizzirani-Kleiner A. A., Araujo W. L., Raaijmakers J. M. Diversity of cultivated endophytic bacteria from sugarcane: genetic and biochemical characterization of Burkholderia cepacia complex isolates. Applied and Environmental Microbiology. 2007;73(22):7259–7267. doi: 10.1128/AEM.01222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeshita K., Kikuchi Y. Genomic comparison of insect gut symbionts from divergent Burkholderia subclades. Genes. 2020;11(7):p. 744. doi: 10.3390/genes11070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tavares M., Kozak M., Balola A., Sá-Correia I. Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products. Clinical Microbiology Reviews. 2020;33(3):e00139–19. doi: 10.1128/CMR.00139-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Estrada-de Los Santos P., Palmer M., Chávez-Ramírez B., et al. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (mycetohabitans gen. Nov., and Trinickia gen. Nov.): implications for the evolution of diazotrophy and nodulation in the burkholderiaceae. Genes. 2018;9(8):p. 389. doi: 10.3390/genes9080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vicenzi F. J., Pillonetto M., Souza H. A. P. H. d. M. d., et al. Polyphasic characterisation of Burkholderia cepaciacomplex species isolated from children with cystic fibrosis. Memórias do Instituto Oswaldo Cruz. 2016;111(1):37–42. doi: 10.1590/0074-02760150314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin Y., Zhou J., Zhou J., et al. Genome-based classification of Burkholderia cepacia complex provides new insight into its taxonomic status. Biology Direct. 2020;15(1):p. 6. doi: 10.1186/s13062-020-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ragupathi N. K. D., Veeraraghavan B. Accurate identification and epidemiological characterization of Burkholderia cepacia complex: an update. Annals of Clinical Microbiology and Antimicrobials. 2019;18(1):p. 7. doi: 10.1186/s12941-019-0306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang Y., Hu Z., Chen H., et al. Multilocus sequencing-based evolutionary analysis of 52 strains of Burkholderia pseudomallei in Hainan, China. Epidemiology and Infection. 2018;147:1–6. doi: 10.1017/S0950268818002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tagele S., Kim S., Lee H., Lee Y. Potential of novel sequence type of Burkholderia cenocepacia for biological control of root rot of maize (Zea mays L.) caused by Fusarium temperatum. International Journal of Molecular Sciences. 2019;20(5):p. 1005. doi: 10.3390/ijms20051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romero-Gutiérrez K. J., Dourado M. N., Garrido L. M., et al. Phenotypic traits of Burkholderia spp. associated with ecological adaptation and plant-host interaction. Microbiological Research. 2020;236 doi: 10.1016/j.micres.2020.126451.126451 [DOI] [PubMed] [Google Scholar]
- 110.Bach E., Seger G. D. d. S., Fernandes G. d. C., Lisboa B. B., Passaglia L. M. P. Evaluation of biological control and rhizosphere competence of plant growth promoting bacteria. Applied Soil Ecology. 2016;99:141–149. doi: 10.1016/j.apsoil.2015.11.002. [DOI] [Google Scholar]
- 111.Coulon P. M. L., Groleau M.-C., Déziel E. Potential of the Burkholderia cepacia complex to produce 4-Hydroxy-3-Methyl-2-Alkyquinolines. Frontiers in Cellular and Infection Microbiology. 2019;9:p. 33. doi: 10.3389/fcimb.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Keum Y. S., Lee Y. J., Lee Y. H., Kim J. H. Effects of nutrients on quorum signals and secondary metabolite productions of Burkholderia sp. O33. Journal of Microbiology and Biotechnology. 2009;19(10):1142–1149. doi: 10.4014/jmb.0901.465. [DOI] [PubMed] [Google Scholar]
- 113.Quan C. S., Zheng W., Liu Q., Ohta Y., Fan S. D. Isolation and characterization of a novel Burkholderia cepacia with strong antifungal activity against Rhizoctonia solani. Applied Microbiology and Biotechnology. 2006;72(6):1276–1284. doi: 10.1007/s00253-006-0425-3. [DOI] [PubMed] [Google Scholar]
- 114.Simonetti E., Roberts I. N., Montecchia M. S., Gutierrez-Boem F. H., Gomez F. M., Ruiz J. A. A novel Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium spp. growth. Microbiological Research. 2018;206:50–59. doi: 10.1016/j.micres.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 115.Liu X., Zhu H., Biswas S., Cheng Y.-Q. Improved production of cytotoxic thailanstatins A and D through metabolic engineering of Burkholderia thailandensis MSMB43 and pilot scale fermentation. Synthetic and Systems Biotechnology. 2016;1(1):34–38. doi: 10.1016/j.synbio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Guillouzer S., Groleau M.-C., Déziel E. The complex quorum sensing circuitry of Burkholderia thailandensis is both hierarchically and homeostatically organized. mBio. 2017;8(6):p. 17. doi: 10.1128/mBio.01861-17.e01861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jenul C., Sieber S., Daeppen C., et al. Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nature Communications. 2018;9(1):p. 1297. doi: 10.1038/s41467-018-03690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Orlandelli R. C., Almeida T. T. d., Alberto R. N., Polonio J. C., Azevedo J. L., Pamphile J. A. Antifungal and proteolytic activities of endophytic fungi isolated from Piper hispidum Sw. Brazilian Journal of Microbiology. 2015;46(2):359–366. doi: 10.1590/S1517-838246220131042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meinhardt L. W., Rincones J., Bailey B. A., et al. Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao: what’s new from this old foe? Molecular Plant Pathology. 2008;9(5):577–588. doi: 10.1111/j.1364-3703.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.dos Santos E. C., Pirovani C. P., Correa S. C., Micheli F., Gramacho K. P. The pathogen Moniliophthora perniciosa promotes differential proteomic modulation of cacao genotypes with contrasting resistance to witches’ broom disease. BMC Plant Biology. 2020;20(1):p. 1. doi: 10.1186/s12870-019-2170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Almeida Lopes K. B., Carpentieri-Pipolo V., Fira D., et al. Screening of bacterial endophytes as potential biocontrol agents against soybean diseases. Journal of Applied Microbiology. 2018;125(5):1466–1481. doi: 10.1111/jam.14041. [DOI] [PubMed] [Google Scholar]
- 122.Zhao D.-L., Wang D., Tian X.-Y., Cao F., Li Y.-Q., Zhang C.-S. Anti-phytopathogenic and cytotoxic activities of crude extracts and secondary metabolites of marine-derived fungi. Marine Drugs. 2018;16(1):p. 36. doi: 10.3390/md16010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajwade J. M., Chikte R. G., Paknikar K. M. Nanomaterials: new weapons in a crusade against phytopathogens. Applied Microbiology and Biotechnology. 2020;104(4):p. 1437. doi: 10.1007/s00253-019-10334-y. [DOI] [PubMed] [Google Scholar]
- 124.Hubert J., Fourrier C., Laplace D., Ioos R. First report of pineapple black rot caused by Ceratocystis paradoxa on Ananas comosus in French guiana. Plant Disease. 2014;98(11):p. 1584. doi: 10.1094/PDIS-05-14-0510-PDN. [DOI] [PubMed] [Google Scholar]
- 125.Fávaro L. C. d. L., Sebastianes F. L. d. S., Araújo W. L. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0036826.e36826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.da Silveira A. P. D., Iório R. d. P. F., Marcos F. C. C., et al. Exploitation of new endophytic bacteria and their ability to promote sugarcane growth and nitrogen nutrition. Antonie Van Leeuwenhoek. 2019;112(2):283–295. doi: 10.1007/s10482-018-1157-y. [DOI] [PubMed] [Google Scholar]
- 127.Niu X., Pei M., Liang C., et al. Genetic transformation and green fluorescent protein labeling in Ceratocystis paradoxa from coconut. International Journal of Molecular Sciences. 2019;20(10):p. 2387. doi: 10.3390/ijms20102387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson M., Raizada M. Fungal pathogens of maize gaining free passage along the silk road. Pathogens. 2018;7(4):p. 81. doi: 10.3390/pathogens7040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Hatmi A., de Hoog G. S., Meis J. F. Multiresistant Fusarium pathogens on plants and humans: solutions in (from) the antifungal pipeline? Infection and Drug Resistance. 2019;12:3727–3737. doi: 10.2147/IDR.S180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alves D. R., Morais S. M., Tomiotto-Pellissier F., et al. Leishmanicidal and fungicidal activity of lipases obtained from endophytic fungi extracts. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0196796.e0196796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hrydziuszko Z., Strub D. J., Labus K., Bryjak J. Burkholderia cepacia lipase immobilization for hydrolytic reactions and the kinetic resolution of the non-equimolar mixtures of isomeric alcohols. Bioorganic Chemistry. 2019;93 doi: 10.1016/j.bioorg.2019.01.041.102745 [DOI] [PubMed] [Google Scholar]
- 132.Lindoso J. A. L., Costa J. M. L., Queiroz I. T., Goto H. Review of the current treatments for leishmaniases. Research and Reports in Tropical Medicine. 2012;3:69–77. doi: 10.2147/RRTM.S24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elkhayat E. S., Ibrahim S. R., Mohamed G. A., Ross S. A. Terrenolide S, a new antileishmanial butenolide from the endophytic fungus Aspergillus terreus. Natural Product Research. 2016;30(7):814–820. doi: 10.1080/14786419.2015.1072711. [DOI] [PubMed] [Google Scholar]
- 134.de Almeida T. T., Ribeiro M. A. D. S., Polonio J. C., et al. Curvulin and spirostaphylotrichins R and U from extracts produced by two endophytic Bipolaris sp. associated to aquatic macrophytes with antileishmanial activity. Natural Product Research. 2018;32(23):2783–2790. doi: 10.1080/14786419.2017.1380011. [DOI] [PubMed] [Google Scholar]
- 135.Rahman L., Shinwari Z. K., Iqrar I., Rahman L., Tanveer F. An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Annals of Clinical Microbiology and Antimicrobials. 2017;16(1):p. 53. doi: 10.1186/s12941-017-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toghueo R. M. K. Anti-leishmanial and anti-inflammatory agents from endophytes: a review. Natural Products and Bioprospecting. 2019;9(5):311–328. doi: 10.1007/s13659-019-00220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kandel S. L., Firrincieli A., Joubert P. M., et al. An In vitro study of biocontrol and plant growth promotion potential of Salicaceae endophytes. Frontiers in Microbiology. 2017;8:p. 386. doi: 10.3389/fmicb.2017.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaudhary R., Balhara M., Jangir D., et al. The Sesamum indicum rhizosphere associated bacterium: a source of antifungal compound. Current Topics in Medicinal Chemistry. 2018;18(1):88–97. doi: 10.2174/1568026618666180206102140. [DOI] [PubMed] [Google Scholar]
- 139.Durairaj K., Velmurugan P., Park J. H., et al. Characterization and assessment of two biocontrol bacteria against Pseudomonas syringae wilt in Solanum lycopersicum and its genetic responses. Microbiological Research. 2018;206:43–49. doi: 10.1016/j.micres.2017.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length 16S rDNA gene of B. cepacia COPS strain compared to reference sequences of Bcc members. Supplementary Figure 2: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length atpD gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 3: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length gltB gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 4: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length gyrB gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 5: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length lepA gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 6: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length phaC gene of B. cepacia COPS strain compared to reference sequences of Bcc members. Supplementary Figure 7: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length recA gene of B. cepacia COPS strain compared to reference sequences of Bcc. Supplementary Figure 8: consensus tree obtained from a neighbor-joining phylogenetic analysis using the Jukes-Cantor method (bootstrap with 1000 replicates) based on full length trpB gene of B. cepacia COPS strain compared to reference sequences of Bcc.
Data Availability Statement
The data used to support the findings of this study are found in laboratory notebooks of Laboratory of Microbiology and Biomolecules (LaMiB), Department of Morphology and Pathology, Federal University of São Carlos, Brazil.


