Figure 4.
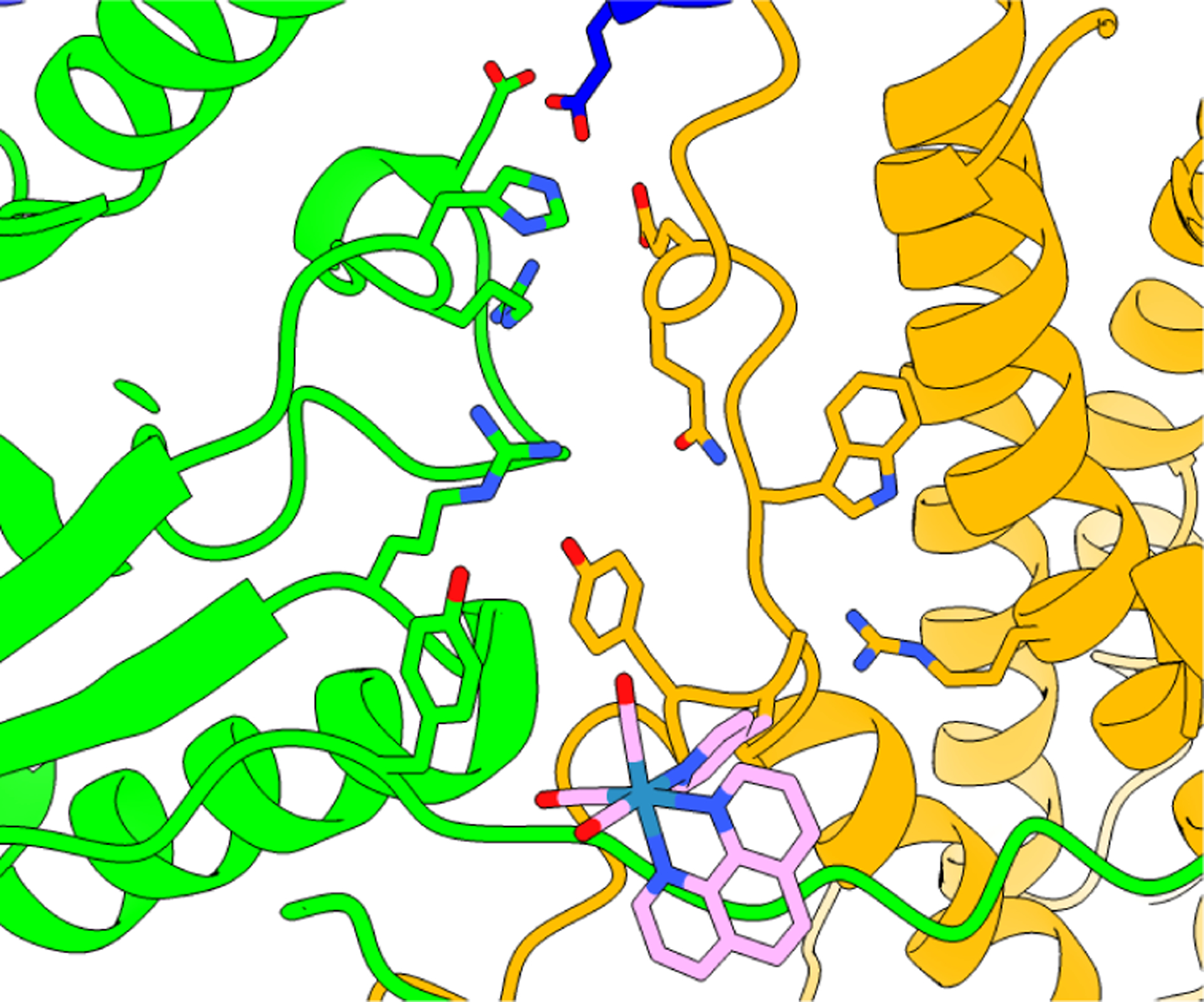
Docking model for the [Re] photooxidant within the α2:β2 interface based on the crystal structure of the [Re] complex and the cyro-EM structure of the active α2β2 E. coli RNR.8 Docking and structural refinement were performed by moving the [Re] unit so as to minimize steric contact of the chromophore and protein sidechains as much as possible, yet steric clashes do exist. This docking model is not intended to be an authentic representation of the actual structure of the complex, but it does provide a general perspective on the location of the S355C labeling site relative to the E52(Q) residue. The [Re] chromophore resides on the opposite side of Y356 relative to the proposed polar channel of H+ release.
