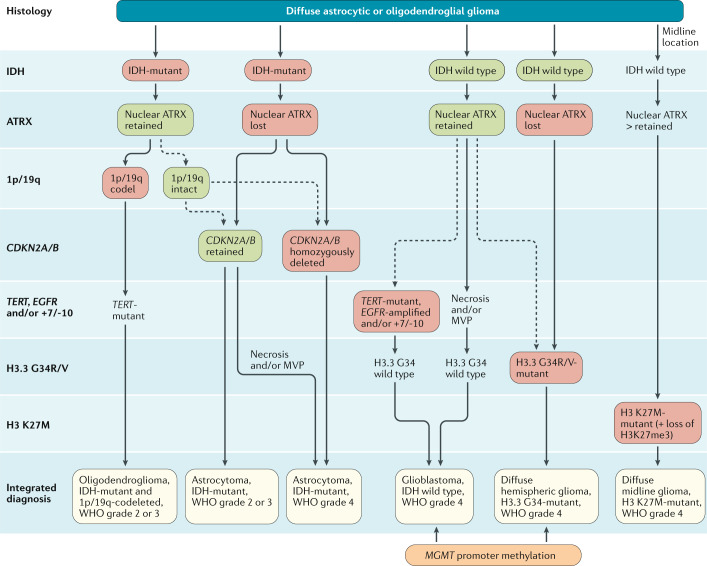
Fig. 1. Diagnostic algorithm for the integrated classification of the major diffuse gliomas in adults.
Tissue specimens obtained through biopsy sampling in patients with diffuse gliomas are routinely assessed by immunohistochemistry for the presence of R132H-mutant IDH1 and loss of nuclear ATRX. In patients aged >55 years with a histologically typical glioblastoma, without a pre-existing lower grade glioma, with a non-midline tumour location and with retained nuclear ATRX expression, immunohistochemical negativity for IDH1 R132H suffices for the classification as IDH-wild-type glioblastoma1. In all other instances of diffuse gliomas, a lack of IDH1 R132H immunopositivity should be followed by IDH1 and IDH2 DNA sequencing to detect or exclude the presence of non-canonical mutations. IDH-wild-type diffuse astrocytic gliomas without microvascular proliferation or necrosis should be tested for EGFR amplification, TERT promoter mutation and a +7/–10 cytogenetic signature as molecular characteristics of IDH-wild-type glioblastomas2. In addition, the presence of histone H3.3 G34R/V mutations should be assessed by immunohistochemistry or DNA sequencing to identify H3.3 G34-mutant diffuse hemispheric gliomas, in particular in young patients with IDH-wild-type gliomas (such as those <50 years of age with nuclear ATRX loss in tumour cells). Diffuse gliomas of the thalamus, brainstem or spinal cord should be evaluated for histone H3 K27M mutations and loss of nuclear K27-trimethylated histone H3 (H3K27me3) to identify H3 K27M-mutant diffuse midline gliomas. The presence and absence of the diagnostically most relevant molecular alterations for each tumour type are highlighted with red and green boxes. MVP, microvascular proliferation.