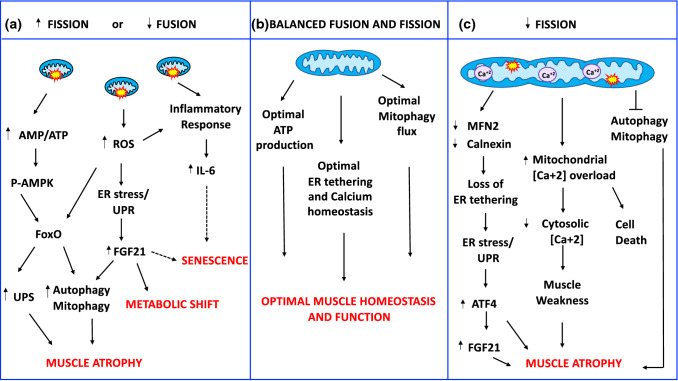
Fig. 2.
Mitochondria-derived signaling pathways controlling muscle mass and whole-body homeostasis. a Increased fission or decreased fusion leads to dysfunctional fragmented organelles, which activate the energy sensor AMPK by increasing the AMP/ATP ratio, ROS production, and the inflammatory response. P-AMPK directly phosphorylates FoxO3 increasing its transcriptional activity and affecting muscle mass. ROS production causes endoplasmic reticulum (ER) stress and activation of unfolded protein response (UPR). UPR induces the ATF4-dependent upregulation of FGF21 secreted by the muscle that contributes to muscle loss, causes a systemic metabolic shift, and premature senescence. b Balanced mitochondrial fusion and fission are critical for muscle function and whole-body homeostasis. c A reduction of mitochondrial fusion results in the accumulation of elongated dysfunctional mitochondria resulting in mitophagy impairment, loss of ER tethering, ER stress, increased mitochondrial calcium overload, and decreased cytosolic calcium causing cell death, muscle loss, and weakness. Dashed lines indicate mechanisms that need more studies