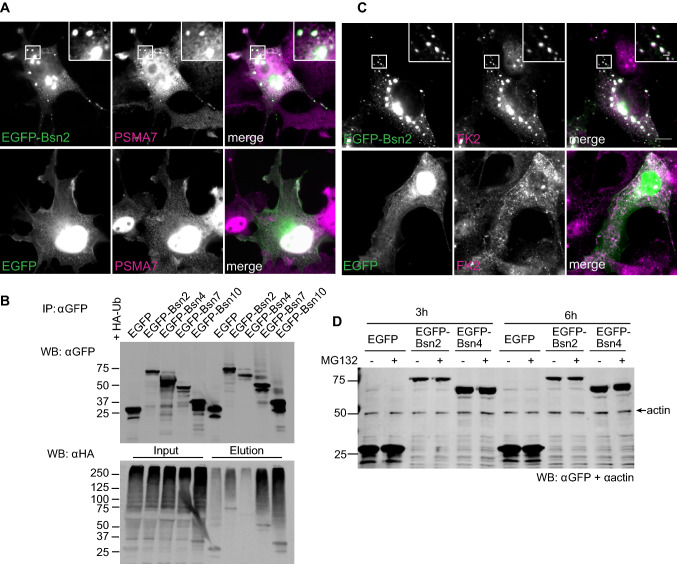
Fig. 3.
Bsn interacts with but it is not degraded by the endogenous proteasome. Endogenous proteasome subunit PSMA7 (a) and ubiquitin (c, stained with FK2 antibody) are recruited to EGFP-Bsn2-containing clusters in COS7 cells. Regions in squares are shown magnified in the inset in the upper left corner. Scale bars represent 10 μm in overview and 2 μm in inset. b EGFP-containing fragments were immunoprecipitated with specific GFP antibodies from HEK293T cells expressing EGFP, EGFP-Bsn2 or EGFP-Bsn4 together with HA-Ub. The expressed (Input) and co-precipitated (Elution) EGFP-fragments and therewith associated Ub moieties were detected by immunoblotting. Co-IP experiments were conducted three to four times on independent HEK293T cell cultures. Input samples confirm expression of all transfected constructs. d HEK293T cells were transfected with EGFP, EGFP-Bsn2 or EGFP-Bsn4 and treated with either 10 µM MG132 to block proteasome or DMSO for 3 or 6 h. Actin serves as a loading control. No differences in protein abundance were observed between conditions for all constructs