Figure 3 |. K63 chain forming HECT E3 ligases show strong preferences for a native lysine acceptor on ubiquitin.
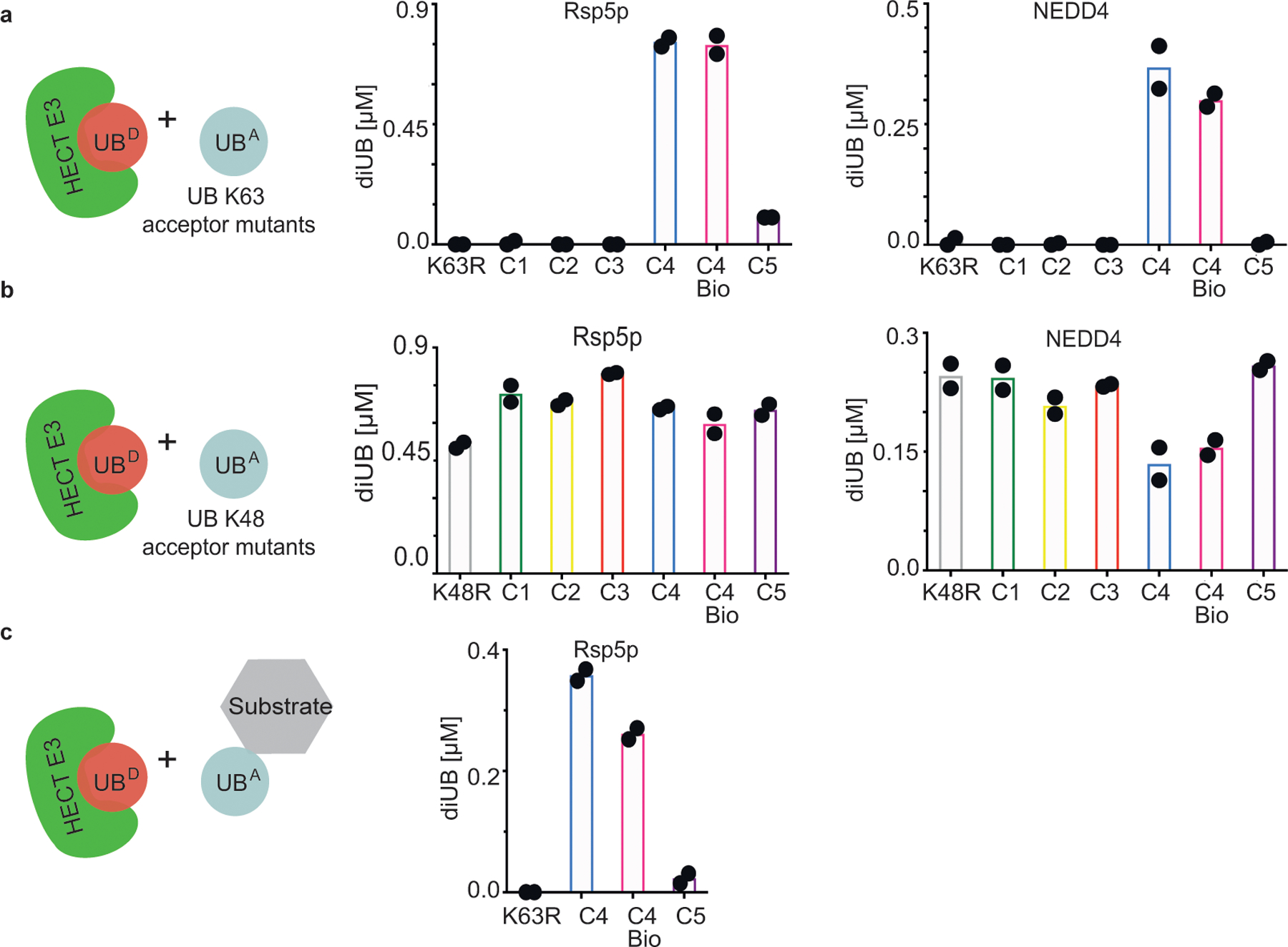
(a) Cartoon of experimental scheme (left), monitoring reactivity of the yeast HECT E3 Rsp5p (middle) or human HECT E3 NEDD4 (right) and formation of di-UB chains with K63UBC1-C5 acceptors (UBA). (b) same as (a), except with K48UBC1-C5 acceptors. (c) HECT E3 ligase-dependent di-UB forming activity in the context of an Rsp5p-bound substrate (sortase-mediated UB, UB K63R, or K63UBC5 linkage to the WW-domain-binding PPPY degron motif of the substrate Sna4p. For all plots, di-UB levels (μMol) represent the final time-points from the reactions (Source Data Fig.3), N=2 independent experiments. For samples derived from the same experiment, gels were processed in parallel.
