Fig. 2.
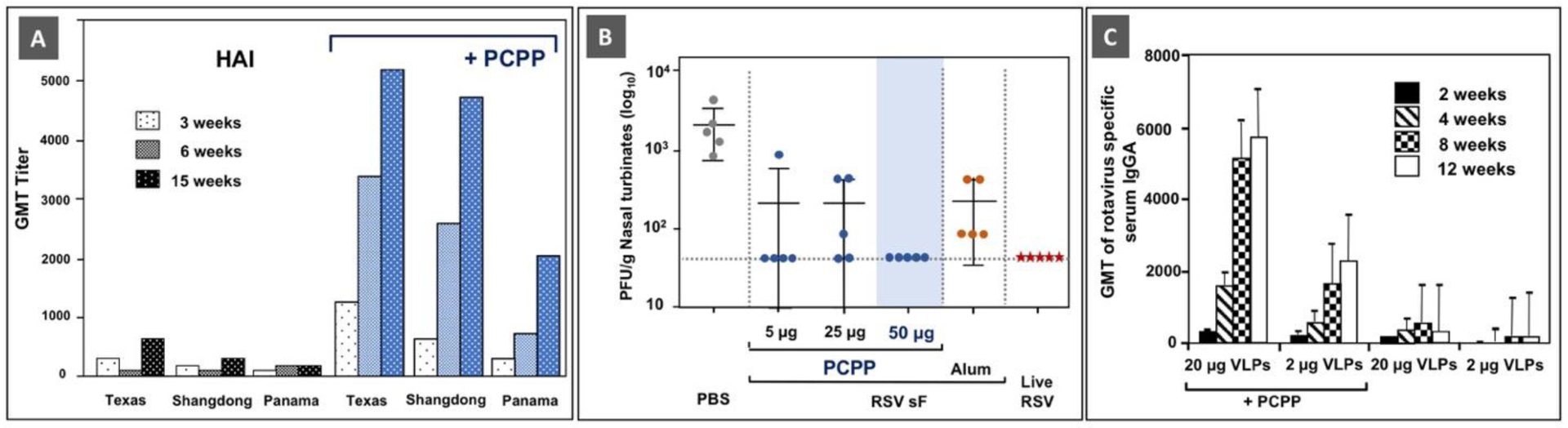
Immunoadjuvant activity of PCPP: (A) The effect of PCPP on hemagglutination-inhibition (HAI) antibody titers to trivalent influenza vaccine (A/Texas/36/91 (H1N1), A/Shangdong/9/93 (H3N2), B/Panama/45/90) in BALB/C mice. Reprinted from [27], Copyright 1997, with permission from Elsevier. (B) Nasal turbinates viral loads 4 days post-challenge with RSV A2 in mice. Infectious virus was detected by Plaque Assay on HEp-2 cells. Adapted with permission from [7]. Copyright 2017, American Chemical Society. (C) Rotavirus-specific serum IgA antibodies after intramuscular immunization with rotavirus VLPs with or without PCPP. Significantly higher IgA titers were observed in mice that received PCPP adjuvanted formulations (p < 0.002). (Reprinted from [32], Copyright 2008, with permission from Elsevier).
