Fig. 6.
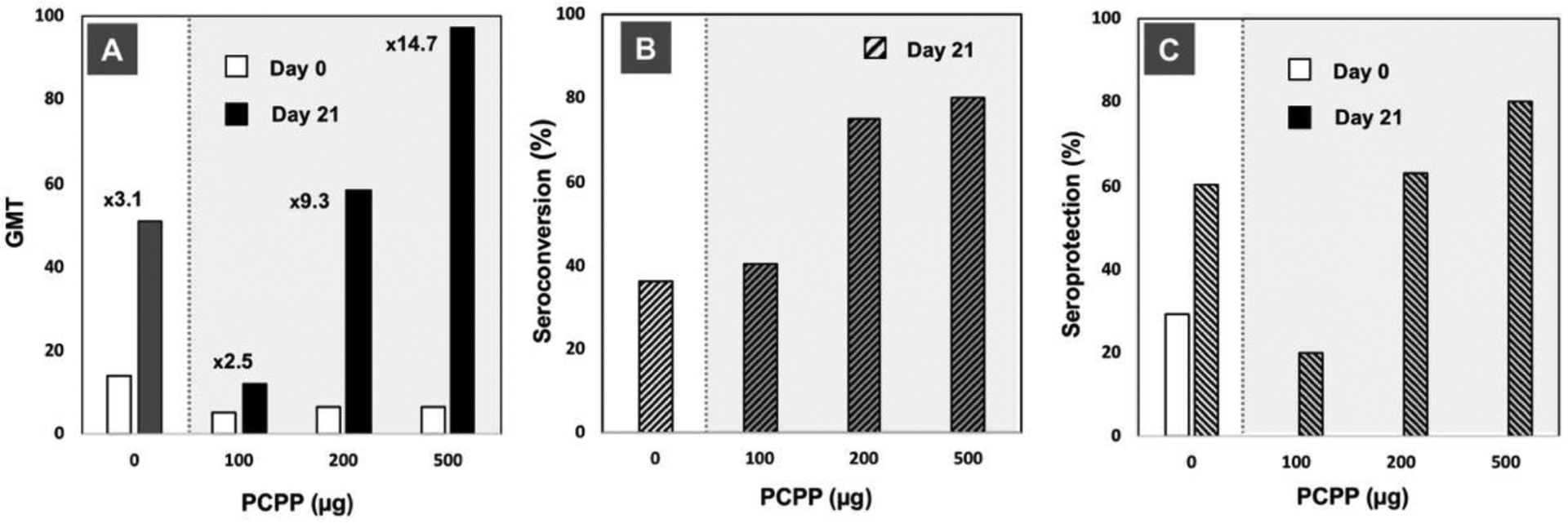
Immunoadjuvant activity of PCPP in clinical trials with influenza vaccine: (A) geometric mean titers (GMT) of A/H3N2 strain specific immune response (numbers indicate fold-increase in GMTs compared to day 0); (B) seroconversion, and (C) seroprotection data versus PCPP dose (strain-specific immunogenicity for A/Johannesburg/33/94 (H3N2) influenza, days 0 and 21 results are shown, 96 subjects). Figures plotted based on the data published in [38].
