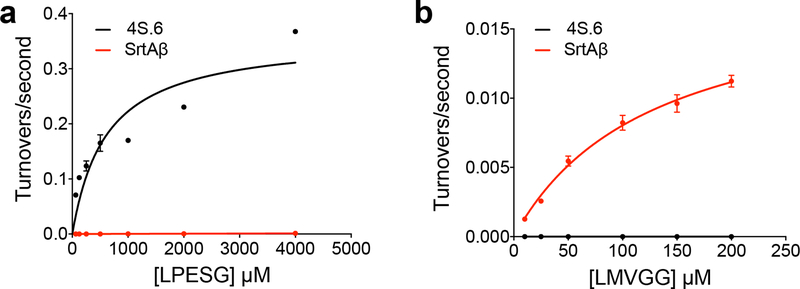
Extended Data Fig. 6: Kinetic analysis of sortase 4S.6 and SrtAβ.
(a) Sortase 4S.6 shows kcat = 0.36 s−1 (95% CI = 0.22 to 0.96 s−1) and KM = 610 μM (95% CI = 90 to 5550 μM) on its cognate substrate LPESG in an established HPLC assay for sortase transpeptidation activity. SrtAβ activity was detectable in this assay, but at levels too low to accurately determine its kinetic parameters on LPESG. Attempts to establish kinetic parameters for LMVGG in the HPLC assay were complicated by this substrate’s limited solubility in the reaction buffer. (b) A more sensitive fluorescence method was used to detect smaller amounts of turnover at lower substrate concentrations. Importantly, the solubility of the LMVGG substrate is within a range where inner filter quenching effects can be corrected. Using this method, SrtAβ shows kcat = 0.018 s−1 (95% CI = 0.015 to 0.023 s−1) and KM = 128 μM (95% CI = 87 to 198 μM) on LMVGG. Sortase 4S.6 activity on LMVGG was not detectable in this assay. Points and error bars represent the average of three replicates ± one standard deviation.