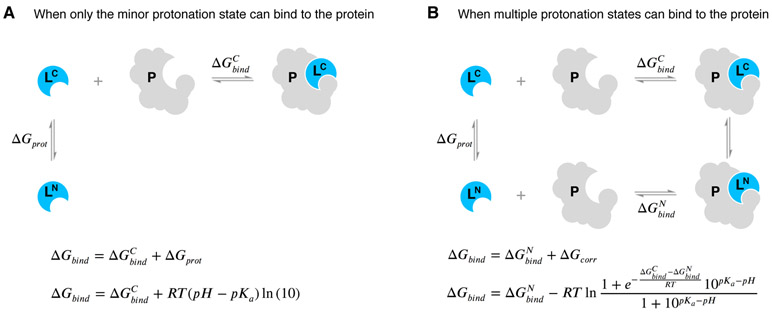
Figure 11. Aqueous ligand pKa can influence overall protein-ligand binding affinity.
A When only the minor aqueous protonation state contributes to protein-ligand complex formation, the overall binding free energy (ΔGbind) needs to be calculated as the sum of binding affinity of the minor state and the protonation penalty of that state. B When multiple charge states contribute to complex formation, the overall free energy of binding includes a multiple protonation states correction (MPSC) term (ΔGcorr). MPSC is a function of pH, aqueous pKa of the ligand, and the difference between the binding free energy of charged and neutral species .