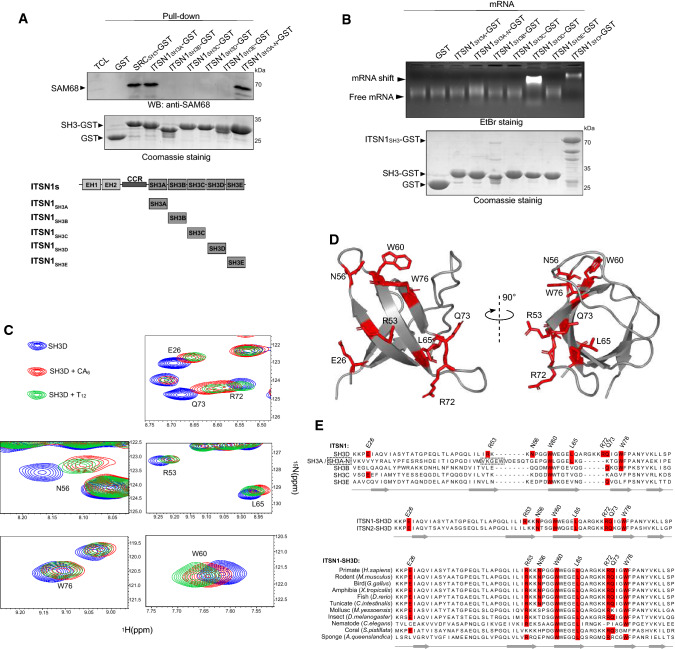
Fig. 4.
ITSN1 interacts with SAM68 and RNA via two different SH3 domains. a GST-pull-down assay revealing the interaction between ITSN1 SH3A domain and SAM68 in vitro. GST was used as a negative control, whereas SH3 domain of SRC kinase was used as a positive control. Immobilized GST-fused SH3 domains were used to precipitate SAM68 from HEK cell lysates. Proteins were visualized using Coomassie staining and Western blotting with anti-SAM68 antibody. b RNA mobility shift assay demonstrating the direct interaction between RNA and ITSN1 SH3D domain. Purified GST-fused ITSN1 SH3 domains or ITSN1SH3 were incubated with 0.4 pmol of 2Luc mRNA and resolved in agarose gel (upper panel). The same amounts of protein samples were resolved via SDS-PAGE and visualized using Coomassie (lower panel). c Two-dimensional 1H-15N SOFAST-HMQC spectra of ITSN1 SH3D domain in the presence of indicated ssDNA oligonucleotides (CA6 or T12). Eight residues with the major chemical shift perturbations were selected and are shown. d Structure of ITSN1 SH3D domain. Amino acid residues indicated on the 1H-15N SOFAST-HMQC spectra are shown and labeled. PDB structure 6GBU was used for the visualization. e Protein alignments showing the conservation of indicated amino acid residues in five ITSN1 SH3 domains (upper panel), ITSN1 and ITSN2 SH3D domains (middle panel), and SH3D domains of ITSN1 homologues in animals (lower panel)