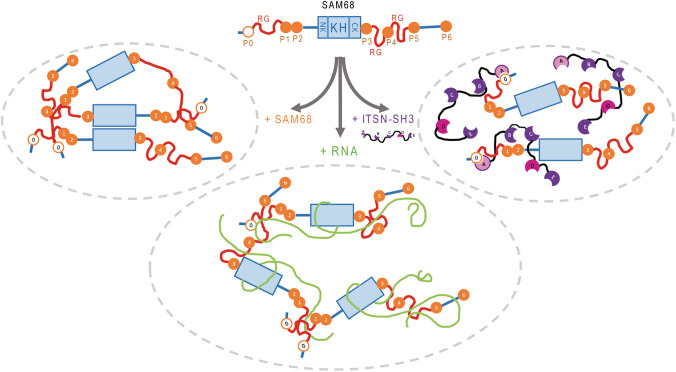
Fig. 6.
Schematic representation of the interplay between scaffold protein ITSN1 and RNA-binding protein SAM68. Due to the presence of RG repeats and KH domain that promote protein oligomerization and phase separation, SAM68 tends to form insoluble aggregates in vitro and SNBs in cells. Increasing the amount of mRNA favors the solubilization of SAM68, since RG repeats and KH domain are involved in mRNA binding. Stimulated by the interaction between SH3D and mRNA, ITSN1 promotes the solubilization of SAM68 through the interaction between ITSN1 SH3A and SAM68 P0