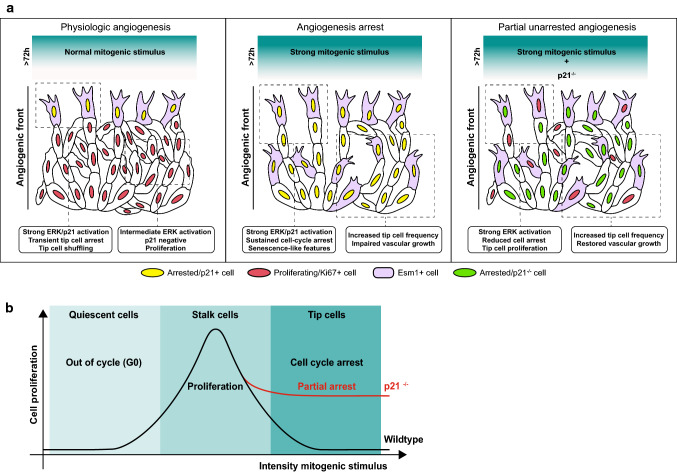
Fig. 3.
The role of mitogenic stimuli and p21 in endothelial cell cycle arrest. a At the angiogenic front, p21+ tip cells arrest and are followed by proliferating Ki67+ stalk cells. If the levels of the mitogenic stimulus are high in all cells, the phenotypic differences between tip and stalk cells disappear as most cells at the angiogenic front become arrested and acquire tip-like features. This cell-cycle arrest at the angiogenic front impairs the sustained vascular network growth despite an increased number of tip cells. If p21 function is lost or inhibited, a high mitogenic stimulus fails to induce cell-cycle arrest in some cells, resulting in the partial preservation of proliferation at the angiogenic front. b The relation of EC proliferation to the intensity of a mitogenic stimulus corresponds to a bell-shaped curve. Depending on the intensity of mitogenic stimulus, cells can be either quiescent, proliferate, or become arrested. EC proliferation is maximal at intermediate mitogenic stimulus. Proliferating ECs become arrested if a threshold is exceeded which triggers the upregulation of p21 and inhibition of proliferation. This hypermitogenic cell cycle arrest can be partially prevented by p21 deletion in ECs, resulting in proliferative activity even at high mitogenic stimulus levels