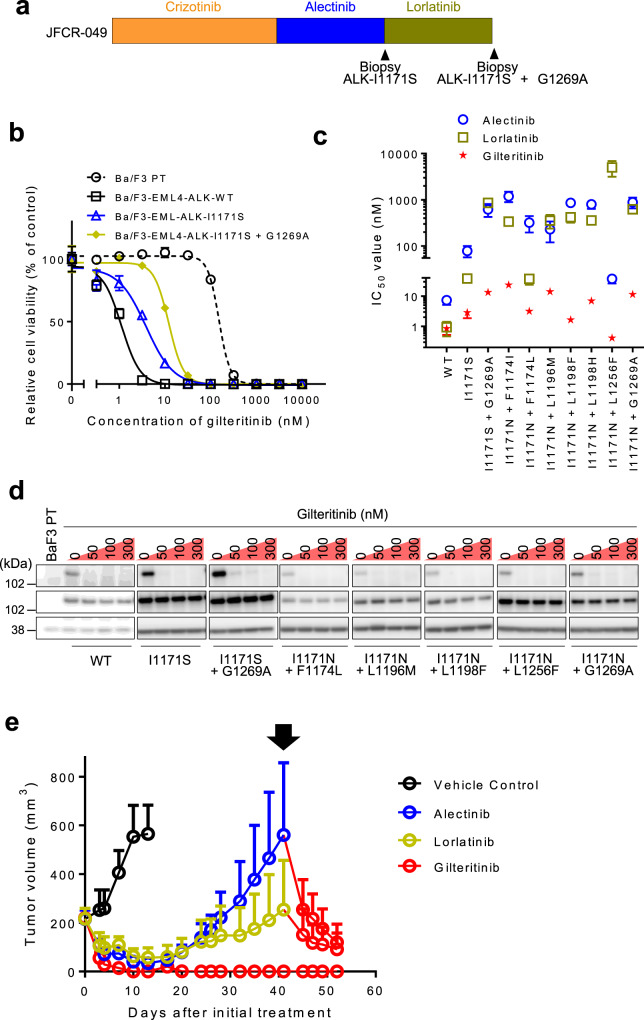
Fig. 4. Efficacy of gilteritinib against EML4-ALK I1171N compound mutants.
a Schematic of the timing of ALK-TKI treatment and biopsy for patients from whom the JFCR-049. b The inhibitory activity of gilteritinib in indicated EML4-ALK expressing Ba/F3 cells. Cells were treated with inhibitors for 72 h and analyzed cell viability using the CellTiter-Glo assay. N = 3 independent samples examined over three independent experiments and representative experiment data are presented as mean values ± SD. c IC50 calculated from the viability analysis of Ba/F3 cells carrying indicated compound mutations. Cells were treated with lorlatinib, alectinib, or gilteritinib for 72 h. N = 3 independent samples examined over three independent experiments and data presented as mean values ± SD. d The suppression of phospho-ALK in Ba/F3 cells carrying single and compound mutations conferring resistance to second-generation or third-generation ALK-TKIs was evaluated via western blotting. Cells were treated with the indicated concentrations of gilteritinib for 3 h (n = 2). e EML4-ALK I1171N + F1174I expressing JFCR-028-3 cells were subcutaneously transplanted into BALB/c nu/nu mice. When the average tumor volume reached ~200 mm3, the mice were randomized to treatment with vehicle control, alectinib (30 mg/kg), lorlatinib (5 mg/kg), or gilteritinib (30 mg/kg) treatment group once daily for 5 days/week via oral gavage (n = 6 per treatment group). At day 41, Alectinib or lorlatinib-treated mice were switched to gilteritinib (30 mg/kg) treatment once daily for 5 days/week. Tumor volumes were measured three times a week. Data are presented as mean values ± SD.