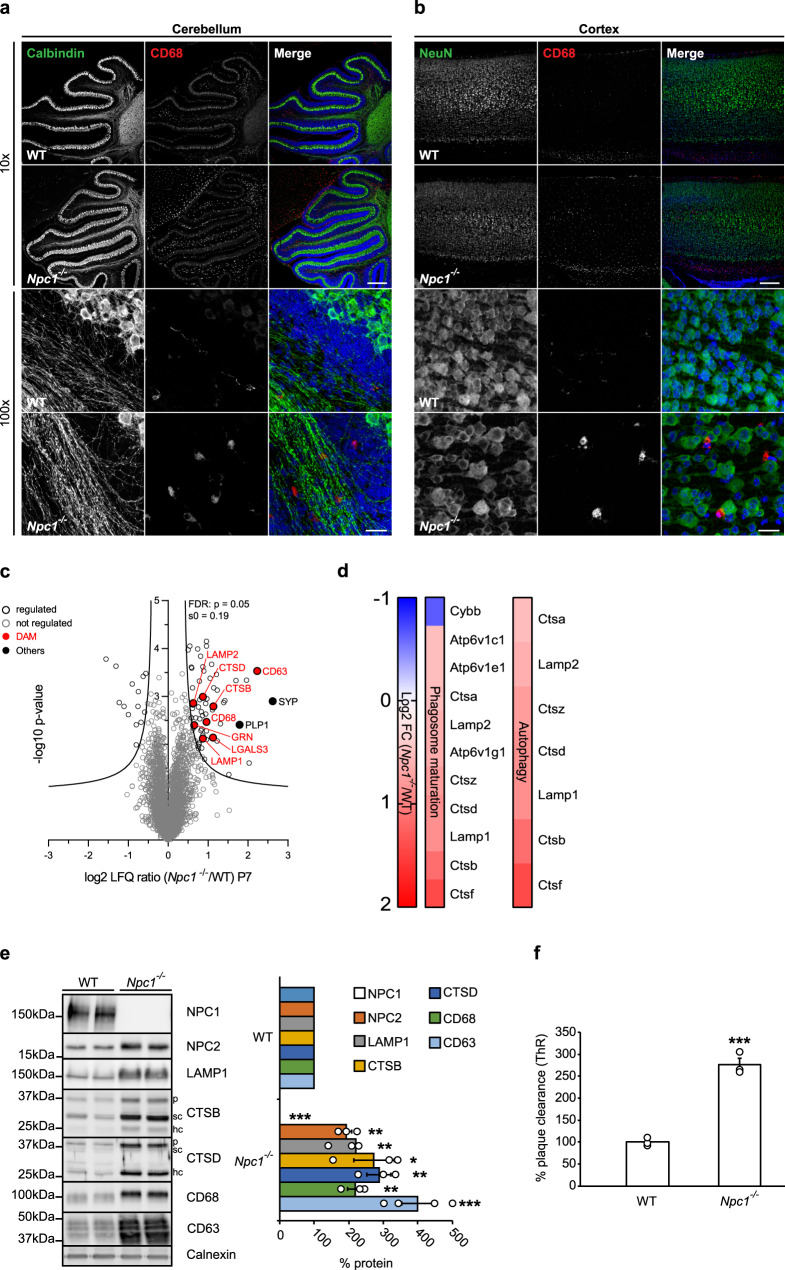
Fig. 2. Switch in microglial proteomic signatures and increased phagocytosis occur already in pre-symptomatic Npc1−/− mice and precede neuronal loss.
a, b Immunostaining of cerebellum (a) and cortex (b) of P7 WT and Npc1−/− mice from three independent experiments (n = 3) using antibodies against neuronal markers (green) Calbindin (Purkinje cells, cerebellum), NeuN (cortex) and lysosomal microglial marker CD68 (red) reveal no neurodegeneration in Npc1−/− mice (10×, upper panels). In contrast, CD68 immunoreactivity showed activated Npc1−/− microglia with amoeboid morphology already at this early pathological stage (100×, lower panels). Hoechst was used for nuclear staining (blue). Scale bars: 250 μm (10×, upper panels) and 25 μm (100×, lower panels). c Proteome analysis of acutely isolated microglia from P7 WT and Npc1−/− littermates. The negative log10 transformed p-value of each protein is plotted against its average log2 transformed LFQ ratio between Npc1−/− and WT microglia. The hyperbolic curves indicate the threshold for a permutation-based FDR correction for multiple hypotheses. Significantly changed proteins (p-value less than 0.05 and FDR corrected) with a log2 fold change larger than 0.5, or smaller than −0.5 are encircled in black (regulated) and not regulated proteins are encircled in gray. Selected DAM proteins (upregulated) are highlighted in red and the synaptic protein synaptophysin (SYP) and myelin protein (PLP1) in black (others). Microglia were analyzed from three independent experiments (n = 3). d Npc1 deletion affects phagosome maturation and autophagy already at pre-symptomatic phase. The heatmap shows the average log2 transformed LFQ ratio between Npc1−/− and WT microglia of all significantly regulated proteins involved in phagosome maturation and autophagy according to IPA. e Validation of MS results via western blot analysis and corresponding quantification. Representative immunoblots of acutely isolated microglia from WT mice and Npc1−/− littermates showing increased level of late endosomal/lysosomal markers (NPC2, LAMP1, CTSB, CTSD, CD68, and CD63). Calnexin was used as a loading control. For cathepsins: p = pro-form; sc = single chain form; hc = heavy chain form. Proteins were quantified by densitometry (ImageJ—NIH) from at least three independent experiments. Values were normalized on WT control and represent mean ± SEM (unpaired two-tailed Student’s t-test). NPC1 (n = 4, p = 3 × 10−6); NPC2 (n = 3, p = 0.004); LAMP1 (n = 3, p = 0.006); CTSB (n = 3, p = 0.04); CTSD (n = 3, p = 0.006); CD68 (n = 3, p = 0.0058); CD63 (n = 4, p = 6 × 10−4). f Npc1−/− microglia from P7 mice already show increased phagocytic capacity toward Aβ. Quantification of Aβ plaque clearance was performed by comparing ThR positive area between a brain section incubated with microglia and the consecutive brain section where no cells were added. Values are expressed as percentages of amyloid plaque clearance normalized to the WT and represent mean ± SEM from three independent experiments (n = 3, p = 0.0003, unpaired two-tailed Student’s t-test).