Table 3.
Oxidative cleavage of alkenes to corresponding aldehydes in the presence of A-CQDs/W catalyst.
| Entry | Structure | Product | Conversion (%)a | Yield (%) b | |
|---|---|---|---|---|---|
| 1 |
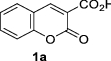
|
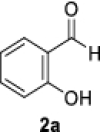
|
95 | 86 | |
| 2 |
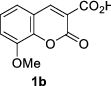
|
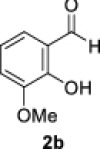
|
78 | 69 | |
| 3c |
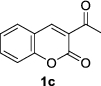
|
|
72 | 63 | |
| 4 |
|

|
100 | 94 | |
| 5 |
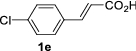
|
|
96 | 89 | |
| 6 |
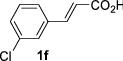
|
|
91 | 84 | |
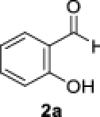
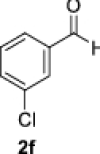
| 7c |

|
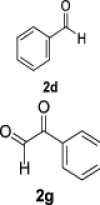
|
83 |
42 38 |
|
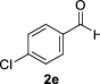
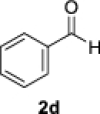
| 8c |
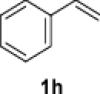
|
|
76 | 63 | |
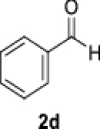
| 9c |

|
|
74 | 74 | |
Reaction conditions: Substrate (1 mmol), H2O2 (3 mmol), H2O (1.5 mL), A-CQDs/W (1 mol %), 3 h, 90 °C.
bConversions were calculated based on initial mmol of substrate.
cIsolated Yields.
dIn the presence of 0.25 mmol CH3COOH.
