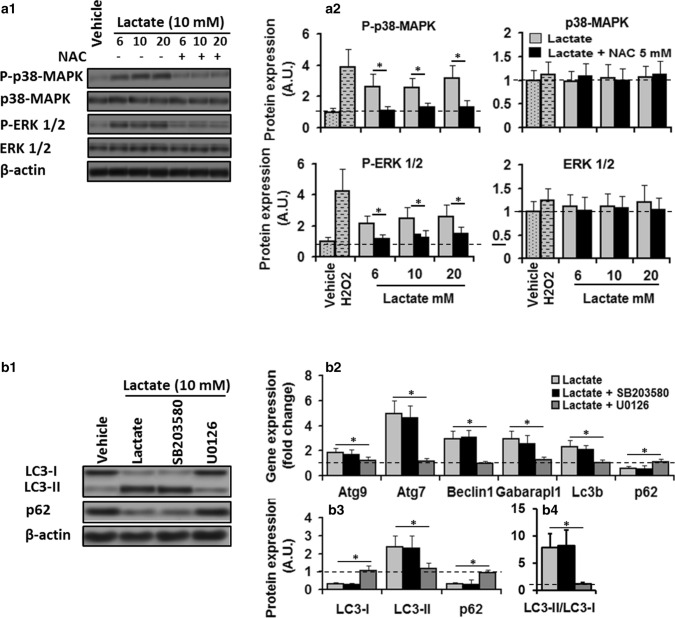
Fig. 2.
Activation of ERK1/2 was required for lactate-induced regulation of autophagy through ROS. a1 In-gel profile for densitometric scanning analysis of p38-MAPK and ERK l/2 and their phosphorylated level in C2C12 myotubes maintained in vehicle or H2O2, or lactate 10 mM for 8 h in the absence or presence of NAC. a2 Lactate increased phosphorylataion of p38-MAPK (two-way ANOVA: main effect of treatment, F = 90.1 p < 0.01; dose × treatment interaction, F = 1.5, p = 0.23) and ERK l/2 (two-way ANOVA: main effect of treatment, F = 113 p < 0.01; dose × treatment interaction, F = 0.86, p = 0.43) compared to control values (dashed lines), but its effects were deterred in the presence of NAC (two-way ANOVA: main effect of treatment, p38-MAPK, F = 56.8, p < 0.01; dose × treatment interaction, F = 0.53, p = 0.59; main effect of treatment, P-Erk1/2, F = 64.2, p < 0.01; dose × treatment interaction, F = 0.44, p = 0.64). b1 In-gel profile and b2–4 bar plots for autophagy-related gene expression and autophagic flux in C2C12 cells maintained in vehicle or lactate (6, 10, and 20 mM, 8 h) in the absence or presence of ERK1/2 inhibitor U0126 (2.5 µM) and p38-MAPK inhibitor SB203580 (10 µM) for 8 h. ERKl/2 inhibition by U0126 abolished the stimulatory effect of lactate on autophagy-related gene expression (one-way ANOVA, all p < 0.01) Atg9, F = 4.7; Atg7, F = 32.8; Beclin 1, F = 31.5; Gabarapl1, F = 13.9; LC3B, F = 18.7; p62, F = 10; all p < 0.01) and autophagic flux (one-way ANOVA: LC3B-I, F = 52; LC3B-II, 12.9; p62, F = 21.7, LC3B-II/LC3B-I, F = 19.3; all p < 0.01), but p38-MAPK inhibition by SB203580 had no effect, suggesting lactate-induced autophagy regulation in C2C12 myotubes is mediated through activation of ERK1/2. * Significant difference between groups (P < 0.01). N = 6 per group