Figure 2. Body Weight–Related Efficacy End Points.
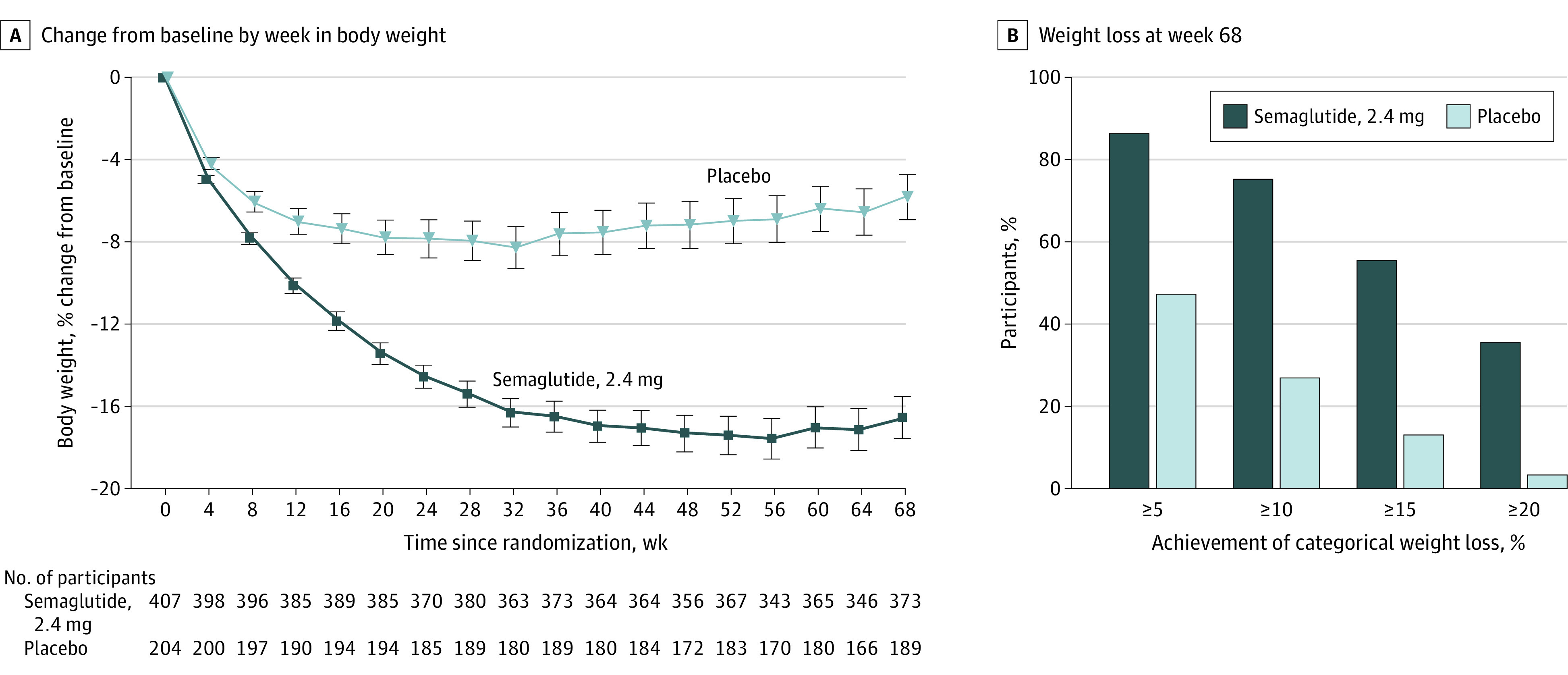
A, The observed mean percentage change in body weight over time for participants in the full analysis set for the in-trial period (from randomization to last contact with the trial site, regardless of treatment discontinuation or rescue intervention). Error bars represent 95% CIs of the mean. B, The observed proportions of participants attaining at least 5% (co–primary end point), 10%, 15%, and 20% reductions in baseline body weight by week 68 in the full analysis set. The proportions shown are cumulative, such that the 88.6% of semaglutide-treated participants who lost more than 5% of baseline body weight includes the 75.3% of participants who lost more than 10%, and so on. See eFigure 4 in Supplement 1 for corresponding on-treatment data (during treatment with the trial product [any dose of trial medication administered within the previous 2 weeks]).
