Abstract
Objective
To determine cross-sectional adherence with the multi-target stool DNA test used for colorectal cancer screening in a large, fully insured Medicare population.
Methods
All patients aged 65–85 with a valid multi-target stool DNA test order from 1 September 2016 to 31 August 2017 identified from the Exact Sciences Laboratories (Madison, WI; sole-source national multi-target stool DNA test provider) database were evaluated for test adherence. Cross-sectional adherence, defined as multi-target stool DNA test completion within 365 days from order date, was analyzed overall and by time to adherence, as well as by available patient (age, sex, test order date, Medicare coverage type) and provider (specialty, year of first multi-target stool DNA test order, multi-target stool DNA test order frequency, and practice location) factors.
Results
Among 368,494 Medicare beneficiaries (64% female), overall cross-sectional adherence was 71%. Cumulative adherence rates increased more rapidly at 30 (44%) and 60 (65%) days, followed by more gradual increases at 90 (67%), 180 (70%), and 365 (71%) days. By provider specialty, primary care clinicians represented a higher percentage of multi-target stool DNA orders than gastroenterologists (88% vs. 6%), but had a lower associated patient adherence rate (71% vs. 78%).
Conclusions
In this large, national sample of Medicare insured older adults, nearly three-quarters of patients adhered with a multi-target stool DNA order for colorectal cancer screening. These real-world data should inform further clinical and population health applications, reimbursement model simulations, and guideline-endorsed colorectal cancer screening strategies adherence.
Keywords: Colorectal neoplasia prevention, Medicare cohort studies, cologuard, patient navigation, colorectal cancer screening
Introduction
Colorectal cancer (CRC) is the fourth most-diagnosed and second deadliest cancer in the United States, with over 145,000 incident and 51,000 fatal cases estimated in 2018.1 Regular participation with average-risk screening has been shown to reduce CRC mortality,2 and the US Preventive Services Task Force (USPSTF) provides a “Grade A” recommendation for CRC screening in average-risk adults aged 50 to 75. Discouragingly, only 63% of screening-eligible adults are up-to-date with CRC screening according to a recent report,3 well below the Healthy People and National Colorectal Cancer Roundtable goals of 70–80% screening adherence.4,5 To realize the full public health potential of CRC screening, further definition of population-based strategies that are both accessible and acceptable is urgently needed.
To facilitate maximum engagement in average-risk CRC screening, guideline review groups such as the USPSTF,2 American Cancer Society,6 National Comprehensive Cancer Network,7 and others have endorsed multiple test options with equal positioning, including the multi-target stool DNA (mt-sDNA) test. Mt-sDNA testing allows for home-based sample collection, with no pre-test requirements for bowel preparation, dietary restriction, or medication adjustment. In addition, the mt-sDNA test is supported by a multilingual (currently over 240 languages) nationwide patient navigation system that includes proactive patient outreach and reminders during the first month after test order receipt. Telephonic assistance is also available for incoming queries (from patients or providers) at any time (24 h/day× 7 days/week × 365 days/year). Results of the mt-sDNA test are reported as “positive” or “negative” based on a composite logistic regression score calculated from the quantitation of 11 molecular biomarkers,8 with performance characteristics for CRC and advanced premalignant lesions previously defined in a large, prospective study.9 Mt-sDNA testing and associated patient navigation support are performed by a sole-source national laboratory (Exact Sciences Laboratories, LLC; Madison, WI; “the laboratory”), enabling ready access to robust, real-world data from a single, centralized repository.
Inadequate patient adherence is a recognized impediment to effective, programmatic CRC screening. The mt-sDNA test was designed to facilitate patient adherence. Mt-sDNA test screening itself does not require any bowel preparation or invasive instrumentation but does provide 24/7 patient and provider support through a comprehensive nationwide telephonic navigation system, plus the consistency and reliability of centralized laboratory analysis. To date, cross-sectional adherence with the mt-sDNA test in a large, national patient sample has not been reported. The primary aim of this retrospective cohort study was to determine cross-sectional adherence with the mt-sDNA test in a defined population of screen-eligible adults. As mt-sDNA testing is a covered benefit for Medicare patients,10 we chose to focus on older adults (aged 65–85), to reduce the potential influence of insurance variability on the outcomes of interest. Associations between cross-sectional adherence and available patient, provider, and other test order attributes were evaluated in secondary analyses to provide further insights regarding clinical application and adoption in the target population.
Methods
Aggregate laboratory data were retrospectively reviewed as part of ongoing laboratory quality management processes and in compliance with the Health Insurance Portability and Accountability Act.
Eligible study participants included Medicare beneficiaries, aged 65 to 85, with a valid mt-sDNA test order received by the laboratory between 1 September 2016 and 31 August 2017. Valid mt-sDNA test orders had to include all information required for the laboratory to accept, perform, and report a test result. De-identified test order attributes extracted from the laboratory database included patient age, sex, test order date, and Medicare coverage type (traditional vs. Medicare Advantage Plan),11 as well as provider specialty, year of first mt-sDNA test order, mt-sDNA test order frequency, and practice location. No mt-sDNA test outcome data are presented.
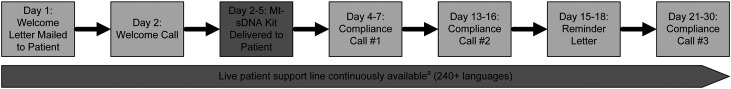
Patient navigation support, including reminders, has been shown to increase CRC screening rates.12–14 All mt-sDNA test orders are supported by a patient navigation system, comprised of outbound phone calls, mailed letter reminders, and a continuously available service center staffed by live personnel with communication capabilities in over 240 languages (Figure 1). The standard navigation protocol begins with a telephone call counseling the patient on the mt-sDNA test and sample collection process, before the laboratory ships the sample collection kit to the patient’s preferred address. Up to two phone call reminders and a final mailed letter reminder are delivered within approximately 30 days following the test order, and reminders are discontinued after this final reminder or upon receipt of a completed test kit at the laboratory. Service specialists are also available to receive inbound calls 24 h/day, 7 days/week, 365 days/year to answer questions and offer additional support for test completion, regardless of time since test order.
Figure 1.
Approximate timing of patient navigation activities following the receipt of an order for the multi-target stool DNA test for colorectal cancer screening at the laboratory. Note: Patient contact and response are variable and generally occur within the day range provided.
Mt-sDNA: multi-target stool DNA.
aThe live support line is available 24 h per day, 7 days per week, 365 days per year and includes a provider support function for healthcare staff. Navigation staff fluent in more common languages are complemented by third-party telephonic, real-time translation support.
Cross-sectional adherence was defined as the percent of eligible participants with a valid mt-sDNA test order who successfully completed the test (positive or negative result) within 365 days from the order date. This time period is consistent with reports of other stool-based CRC screening tests, such as the fecal immunochemical test (FIT).15–17
Descriptive statistics were used to analyze overall cross-sectional adherence and to further describe associations with patient (age, sex, test order date, and Medicare coverage type) and provider characteristics (practice specialty, year of first mt-sDNA order, mt-sDNA order frequency, and practice location). The median time to adherence (TTA) and interquartile range (IQR) of TTA were calculated for the overall cohort, as well as for relevant subgroups. Provider mt-sDNA test order frequency was analyzed by quartiles, as determined by ranking providers whose first order was prior to the start of the study period by the number of mt-sDNA tests they prescribed within the study period. The provider list was then divided into four groups, each with roughly equal numbers of providers. Utilizing the provider location data, mt-sDNA cross-sectional adherence was further analyzed with respect to states having the highest (Maine 75.9%, Connecticut 75.8%, and Massachusetts 75.3%) vs. the lowest (Mississippi 59.9%, Oklahoma 58.8%, and New Mexico 58.5%) state-level CRC screening rates, as determined by the Centers for Disease Control and Prevention.18
Given the large sample size (368,494 Medicare beneficiaries), the application of traditional biostatistics to compare subgroup adherence outcomes results in statistical significance being reached for nearly every such comparison. For example, with 100,000 cases per subgroup, the 95% confidence interval has a width <0.6% for an observed 70% adherence rate. Also, an operationally insignificant 1% difference in adherence rates (69.5% vs. 70.5%) will achieve a two-sided chi square p-value ∼1 in a million according to a chi square test. Thus, we do not report two-sided 95% confidence intervals for point estimates or two-sided p-values for subgroup comparisons.
Results
A total of 368,494 patients (64% women) were identified who met the defined study criteria. Mean age was 71.9, with the following five-year age distributions: 38.3% ages 65 to 69, 33.0% ages 70 to 74, 19.1% ages 75 to 79, and 9.6% ages 80 to 85. Within the study cohort, monthly mt-sDNA test orders were lowest at the beginning (September 2016; n = 21,429) and highest at the end (August 2017; n = 42,229) of the data collection period. By Medicare coverage type, 68% had Traditional Medicare and 32% had Medicare Advantage.
The majority of mt-sDNA test orders were placed by primary care clinicians (325,394; 88%), with fewer orders placed by gastroenterology (22,210; 6%), obstetrics and gynecology (4014; 1%), and other (16,876; 5%) specialties. Provider’s year of first mt-sDNA order was 2014 for 25,635 (7%) study patients, 2015 for 129,349 (35%) study patients, 2016 for 153,598 (42%) study patients, and 2017 for 59,912 (16%) study patients. Provider mt-sDNA test order frequency was analyzed by quartiles by first ranking providers by the number of mt-sDNA tests they prescribed within the study period, to obtain comparable numbers of providers per quartile. With respect to provider practice location, all 50 states were represented in the study population. Providers located in the three states with the highest (Maine, Connecticut, Massachusetts) and lowest (Mississippi, Oklahoma, New Mexico) state-level CRC screening rates comprised 12,106 (3.3%) and 9413 (2.6%) orders for study patients, respectively.
Completed mt-sDNA test results were obtained for 262,084/368,494 study participants, yielding an overall cross-sectional adherence rate of 71.1% (Table 1). Median (IQR) TTA was 27 (20, 37) days, with cumulative adherence at 30, 60, 90, and 180 days of 43.8%, 64.6%, 67.2%, and 69.7%, respectively. Adherence across patient age groups was similar: 70.7% for ages 65 to 69, 70.9% for ages 70 to 74, 72.3% for ages 75 to 79, and 71.2% for ages 80 to 85. Adherence was also similar when compared by patient sex, test order date, and Medicare coverage type (Table 1).
Table 1.
Cross-sectional adherence with the Mt-sDNA test, by patient-level factors.
| Cross-sectional adherence |
Time to adherence (TTA) |
|||
|---|---|---|---|---|
| Patient-level factor | Mt-sDNA tests ordered, N | Mt-sDNA tests completed, N | % | Median (IQR), days |
| All eligible study patients | 368,494 | 262,084 | 71.1 | 27 (20, 37) |
| Age (y) | ||||
| 65–69 | 141,191 | 99,764 | 70.7 | 28 (21, 39) |
| 70–74 | 121,566 | 86,220 | 70.9 | 27 (20, 37) |
| 75–79 | 70,502 | 50,998 | 72.3 | 25 (20, 35) |
| 80–85 | 35,235 | 25,102 | 71.2 | 24 (19, 34) |
| Gender | ||||
| Women | 235,617 | 166,601 | 70.7 | 28 (21, 38) |
| Men | 132,806 | 95,433 | 71.9 | 26 (20, 35) |
| Medicare coverage type | ||||
| Traditional Medicare | 250,800 | 178,922 | 71.3 | 27 (20, 37) |
| Medicare advantage | 117,694 | 83,162 | 70.7 | 27 (20, 37) |
| Test order date | ||||
| September 2016 | 21,429 | 15,276 | 71.3 | 21 (15, 31) |
| October 2016 | 23,961 | 16,894 | 70.5 | 24 (18, 34) |
| November 2016 | 26,032 | 18,538 | 71.2 | 27 (21, 35) |
| December2016 | 23,408 | 16,474 | 70.4 | 29 (21, 41) |
| January 2017 | 26,894 | 19,591 | 72.8 | 27 (21, 36) |
| February 2017 | 27,161 | 19,821 | 73.0 | 28 (21, 38) |
| March 2017 | 34,339 | 24,553 | 71.5 | 28 (22, 37) |
| April 2017 | 32,532 | 23,343 | 71.8 | 27 (21, 39) |
| May 2017 | 38,921 | 27,674 | 71.1 | 29 (23, 39) |
| June 2017 | 37,898 | 26,745 | 70.6 | 26 (20, 37) |
| July 2017 | 33,690 | 23,636 | 70.2 | 24 (17, 37) |
| August 2017 | 42,229 | 29,539 | 69.9 | 25 (19, 36) |
Mt-sDNA: multi-target stool DNA; TTA: median time to adherence; IQR: interquartile range.
Patients with mt-sDNA tests ordered by gastroenterologists had a higher adherence rate (78.0%) than those ordered by primary care providers (70.7%), obstetricians and gynecologists (73.1%), or other specialists (69.4%). Provider’s year of first mt-sDNA test order was also associated with patient cross-sectional adherence, with providers whose first order was in 2014 having higher patient adherence compared with providers whose first order was in 2017 (72.7% vs. 69.8%, respectively) (Table 2). High or low state-level CRC screening rates including any modality were not associated with patient adherence with the mt-sDNA test (Table 2). Geographic location of ordering providers (zip code) was gathered as a voluntary disclosure and was available for 99.9% of study participants. Among provider zip codes with ≥50 participants receiving mt-sDNA tests (n = 146,334), mean patient adherence within 365 days was 71.2%, similar to the overall population. Lastly, the overall population had a median income of $32,003.
Table 2.
Cross-sectional adherence with the Mt-sDNA test, by provider-level factors.
| Cross-sectional adherence |
Time to adherence (TTA) |
|||
|---|---|---|---|---|
| Provider-level factor | Mt-sDNA tests ordered, N | Mt-sDNA tests completed, N | % | Median (IQR), days |
| All eligible study patients | 368,494 | 262,084 | 71.1 | 27 (20, 37) |
| Practice specialty | ||||
| Gastrointestinal | 22,210 | 17,328 | 78.0 | 25 (20, 34) |
| OB/GYN | 4014 | 2933 | 73.1 | 28 (21, 38) |
| Primary care | 325,394 | 230,107 | 70.7 | 27 (20, 37) |
| Other | 16,876 | 11,716 | 69.4 | 27 (20, 36) |
| Year of first test order | ||||
| 2014 | 25,635 | 18,636 | 72.7 | 27 (20, 36) |
| 2015 | 129,349 | 92,448 | 71.5 | 27 (20, 36) |
| 2016 | 153,598 | 109,190 | 71.1 | 27 (20, 37) |
| 2017 | 59,912 | 41,810 | 69.8 | 27 (20, 38) |
| Test order frequency | ||||
| First quartile | 121,301 | 85,867 | 70.8 | 27 (20, 37) |
| Second quartile | 23,397 | 17,104 | 73.1 | 27 (20, 37) |
| Third quartile | 52,188 | 37,805 | 72.4 | 27 (20, 37) |
| Fourth quartile | 171,608 | 121,308 | 70.7 | 27 (20, 36) |
| Practice location | ||||
| Highest state-level CRC screening ratesa | ||||
| Maine | 616 | 454 | 73.7 | 28 (22, 38) |
| Connecticut | 5469 | 3728 | 68.2 | 28 (21, 39) |
| Massachusetts | 6021 | 4111 | 68.3 | 29 (22, 41) |
| Lowest state-level CRC screening ratesa | ||||
| Mississippi | 2461 | 1725 | 70.1 | 25 (20, 35) |
| Oklahoma | 5247 | 3795 | 72.3 | 26 (20, 36) |
| New Mexico | 1705 | 1237 | 72.6 | 28 (21, 38) |
Mt-sDNA: multi-target stool DNA; TTA: median time to adherence; IQR: interquartile range; CRC: colorectal cancer.
aHighest and lowest state-level CRC screening rates as determined by the Centers for Disease Control and Prevention.18
Discussion
Data from this large, national sample of Medicare-insured older adults demonstrate high cross-sectional adherence (71.1%) for CRC screening with the mt-sDNA test. Adherence rates were comparable across multiple patient-level factors, suggesting broad acceptance of this Food and Drug Administration (FDA)-approved, noninvasive, navigation-supported CRC screening option within the Medicare patient population. Higher mt-sDNA test adherence rates were noted for orders placed by gastroenterologists compared with other provider groups, supporting a hypothesis of a positive influence from disease specialists on CRC screening participation. This study represents the first report of mt-sDNA test adherence rates in a real-world setting and should be informative for near-term application to CRC screening program planning, model simulation, comparative effectiveness studies, and other efforts to optimize CRC screening.
Achieving the full public health potential of CRC screening requires high-level engagement at each step in a multi-stage process. According to published reports, patient preference for stool-based DNA testing compares favorably with other endorsed CRC screening options.19–21 Further, a recent systematic review and meta-analysis of data from 73 randomized, controlled trials identified patient navigation as a key indicator of increased CRC screening completion rates.13 Therefore, it seems likely that the combination of positive perception and embedded navigation support contributed to nearly three-quarters of patients in our study adhering with the mt-sDNA test for CRC screening.
Considering the age of our study cohort (mean (range) 72 (65–85)), it seems reasonable to speculate that other guideline-endorsed CRC screening methods may have been previously declined by, not offered to, or not adhered with by these patients. Similarly, patients in this age cohort may also have participated in CRC screening in the past with another screening modality, did not stay up to date with screening, and returned to screening when a high-sensitivity stool test was offered as an alternative to their previous options. Indeed, based on a random sample of Medicare patients with complete mt-sDNA test results contacted between 1 September 2016 and 31 August 2017 (n = 4452), 40.1% reported no prior CRC screening test (Exact Sciences data), but screening history was not collected for all patients in this study. Screening success by this combination of test and navigation was seen in a separate study of Medicare beneficiaries. Prince et al. found that when individuals who were non-adherent with CRC screening were offered the mt-sDNA test, 88% completed the test, and 96% of those with a positive mt-sDNA test result went on to complete a subsequent diagnostic colonoscopy exam.22 Together, these data emphasize the potential for mt-sDNA testing to facilitate greater participation with CRC screening in older adults, even when past adherence has been less than ideal.
While patient and provider agreement to a mutually selected CRC screening strategy is required, it is often insufficient to ensure test completion. As noted above, patient navigation has been demonstrated to help drive follow-through with healthcare interventions, including CRC screening.12,13 The multilingual patient navigation system is integrated with all mt-sDNA orders, and provides assistance in the form of up-front education, periodic reminders, and ongoing call center support. The mt-sDNA patient navigation system is proactive for approximately 30 days following the initial order receipt, and continuously reactive (i.e. available for inbound queries from patients or providers) during and after that period. These value-added services represent a differentiated feature of the mt-sDNA test to help drive adherence and are provided at no additional cost to the Medicare program. Although this study was not designed to quantify the direct impact of navigation support in driving test completion, we found that the majority (61.5%) of patients who adhered with mt-sDNA testing did so within the first 30 days, when outbound patient navigation efforts are most active. Data from a recent, smaller study of tailored interventions to increase CRC screening in a slightly younger population of non-adherent women (ages 51–74; n = 1196) also showed that telephone counseling, provided as a single phone call prior to mailing of a FIT/Fecal Occult Blood Test (FOBT) test kit, resulted in a significantly increased completion rate (41.7%) compared with no intervention (11.1%).23 In a retrospective longitudinal study of Kaiser Permanente plan members (n = 670,841) who were mailed a “FIT kit” for CRC screening, with additional support in the form of in-person, mail, secure email, and telephone reminders as needed, 48.2% completed the initial round. The kit included the FIT (OC FIT-CHEK; Polymedco), a standardized letter from the patient’s primary care provider, directions for completing and mailing the test, and a preprinted laboratory requisition order form. Outreach included in-person, mail, secure e-mail, and telephone reminders as needed.15 Notably, the navigation support provided with the mt-sDNA test also includes multiform, multi-touch contacts after the test kit is received, along with 24 × 7×365 call center support as needed, which could plausibly be linked to the higher adherence rates observed in our study. While data from the current study were derived solely from United States subjects, improving CRC screening adherence is a global challenge with an international audience. Like the United States, many countries lack a national CRC screening program. Among countries that have implemented programmatic screening, individual participation rates vary widely depending on the screening test(s) employed, national policies, access to other preventive services, and cultural differences.24 As CRC screening with the mt-sDNA test requires a prescription from a healthcare care provider, these results may be of particular interest in those countries where CRC screening is similarly initiated by clinical practitioners.
Longitudinal adherence with mt-sDNA testing cannot yet be meaningfully evaluated, given its relatively recent FDA approval (August 2014), and guideline-recommended screening interval of three years.6,7,25 However, existing data (albeit somewhat limited) referent to repeat participation with other stool-based CRC screening tests, which are recommended annually,26 highlight opportunities for improvement. In a follow-up study of participants assigned to annual FOBT screening in a randomized, controlled trial, Liang et al. found that 14% remained adherent at three years.27,28 A larger retrospective analysis of over 1.1 million patients, seen at 136 Veterans Affairs medical centers, similarly reported that 14.1% of men and 13.7% of women who elected to pursue FOBT-based CRC screening had received at least four FOBTs during the five-year study period.29 More discouragingly, another retrospective analysis of patient-level data (n = 151,638) from a national health claims database (Clinformatics DataMart, affiliated with Optum) revealed that only 0.3% of individuals continuously insured by a single national payer had received yearly FIT/FOBT screening over the 10-year assessment period.29 While annual FIT/FOBT is recommended in the United States, some countries utilize biennial FIT or FOBT in national CRC screening programs.30,31 Biennial screening with FIT in the Netherlands demonstrated an adherence rate of 48% over four rounds of screening.32 While lower than the currently reported cross-sectional adherence for mt-sDNA test screening, further experience is needed to rigorously define longitudinal adherence for the mt-sDNA test given the limited time-frame of clinical availability and the recommended three-year interval between screening examinations.
Our study did not find any clinically relevant associations between mt-sDNA cross-sectional adherence and patient factors, including age, sex, Medicare coverage type, geography, or test order date. On a population basis, mt-sDNA test adherence was similar in states with both higher (>75%) and lower (<60%) overall CRC screening rates,18,33 suggesting that implementation of mt-sDNA screening in states with relatively low CRC screening participation could help to engage broader patient engagement if applied or promoted more vigorously. With respect to provider factors, earlier adopters (as evidenced by year of first mt-sDNA order) had higher test completion rates, regardless of specialty, which may reflect the importance of provider and office staff familiarity with the mt-sDNA test and its embedded patient navigation program. Additionally, practice specialty was associated with higher cross-sectional adherence, with observed rates of 78% vs. 71% for gastroenterologists and primary care providers, respectively. The observed difference in adherence by practice specialty may be due to provider factors (e.g. strength of screening recommendation, office education, and a patient’s appreciation of specialty knowledge) or patient factors (e.g. commitment to screening may be higher in patients completing a referral to gastroenterology), or some combination of both. Further investigation may serve to inform and optimize future CRC screening strategies.
Several limitations of this study are worthy of comment. First, only Medicare beneficiaries were included; however, the sample size was large, and this age group represents a substantial proportion of the average-risk CRC screening target population that has uniform test coverage without out-of-pocket expenses between the ages of 50 to 85, removing test-related financial barriers. Nevertheless, these findings may not be generalizable to the pre-Medicare population. Second, although the mt-sDNA test is FDA-approved for CRC screening in average-risk individuals, we did not have access to sufficient data from the mt-sDNA test to confirm average-risk status for all study participants in this real-world study. Third, mt-sDNA test adherence could only be meaningfully evaluated on a cross-sectional basis, due to the recency of FDA approval and clinical adoption. Assessment of longitudinal adherence is anticipated in future studies. Fourth, because the patient navigation system is automatically engaged following an mt-sDNA test order, independent effects of the mt-sDNA test design features and the navigation support system could not be determined. Lastly, the patient and provider factors analyzed in our study were limited to those characteristics that could be captured from the existing laboratory database. More detailed assessment of factors that could influence mt-sDNA screening adherence, such as socioeconomic status and social determinants of health, is worthy of further investigation. Factors influencing patients and providers to select the mt-sDNA test for CRC screening, such as test awareness, risk of complications from invasive screening tests in older adults, and specific test characteristics, is also of interest in future studies.
Conclusions
Based on data from this retrospective study of a national sample of Medicare insured older adults, high initial CRC screening adherence rates can be achieved with mt-sDNA testing. Features of the mt-sDNA test such as the noninvasive approach, widespread accessibility, and embedded patient navigation system probably contributed to successful test completion, and can be further leveraged to accelerate realization of CRC screening participation targets. Future studies examining similar characteristics in the commercially insured population will be of interest, to confirm and complement the findings currently reported.
Footnotes
Authors’ contribution: E Weiser, PD Parks, BM Berger, and P Limburg contributed to the conception and design of the study. E Weiser, PD Parks, BM Berger, RK Swartz, J Van Thomme, and P Limburg developed the methodology. RK Swartz, J Van Thomme acquired data. E Weiser, PD Parks, RK Swartz, J Van Thomme, PT Lavin, P Limburg, and BM Berger contributed to the analysis and interpretation of data (e.g. statistical analysis). J Van Thomme and PT Lavin performed biostatistics and computational analysis. BM Berger, RK Swartz, E Weiser, PD Parks, and P Limburg contributed to the writing, review, and/or revision of the manuscript. E Weiser, PD Parks, and BM Berger were responsible for study supervision.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study represents a collaboration between investigators at Mayo Clinic and Exact Sciences Corporation (Madison, Wisconsin). Dr. Limburg serves as Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement. Berger is a clinical consultant to and equity holder of Exact Sciences. Weiser, Parks, Swartz, and Van Thomme are employees of Exact Sciences.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by Exact Sciences. Lavin has no financial disclosures. Limburg serves as Chief Medical Officer for Exact Sciences through a contracted services agreement with Mayo Clinic. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement.
ORCID iD: Rebecca K Swartz https://orcid.org/0000-0002-6006-1118
References
- 1.American Cancer Society. Cancer facts & figures 2019. Atlanta: American Cancer Society, 2019. [Google Scholar]
- 2.US Preventive Services Task Force. Screening for colorectal cancer US preventive services task force recommendation statement. JAMA 2016; 315: 11. [DOI] [PubMed] [Google Scholar]
- 3.White A, Thompson TD, White MC, et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep 2017; 66: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Colorectal Cancer Roundtable. 80% pledge. Atlanta: American Cancer Society, 2019. https://nccrt.org/80-2018-pledge/. [Google Scholar]
- 5.Office of Disease Prevention and Health Promotion. Healthy people 2020. Washington, DC: US Department of Health and Human Services, 2018. [Google Scholar]
- 6.American Cancer Society. American cancer society guideline for colorectal cancer screening. Atlanta: American Cancer Society, 2018. [Google Scholar]
- 7.Provenzale D, Gupta S, Ahnen DJ, et al. NCCN guidelines insights: colorectal cancer screening, version 1.2018. J Natl Compr Canc Netw 2018; 16: 939–949. [DOI] [PubMed] [Google Scholar]
- 8.Lidgard GP, Domanico MJ, Bruinsma JJ, et al. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol 2013; 11: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 9.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014; 370: 1287–1297. [DOI] [PubMed] [Google Scholar]
- 10.US Centers for Medicare & Medicaid Services. Your Medicare coverage: multi-target stool DNA tests, 2019. https://www.medicare.gov/coverage/multi-target-stool-dna-tests.
- 11.Centers for Medicare & Medicaid Services. Medicare & you: the official U.S. government Medicare handbook. USA: U.S. Department of Health and Human Services, 2019.
- 12.Rice K, Gressard L, DeGroff A, et al. Increasing colonoscopy screening in disparate populations: results from an evaluation of patient navigation in the New Hampshire Colorectal Cancer Screening Program. Cancer 2017; 123: 3356–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 2018; 178: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin 2011; 61: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016; 164: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielson CM, Vollmer WM, Petrik AF, et al. Factors affecting adherence in a pragmatic trial of annual fecal immunochemical testing for colorectal cancer. J Gen Intern Med 2019; 34: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012; 172: 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Use of colorectal cancer screening tests by state. Atlanta: Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 19.Schroy PC, Lal S, 3rd, Glick JT, et al. Patient preferences for colorectal cancer screening: how does stool DNA testing fare? Am J Manag Care 2007; 13: 393–400. [PubMed] [Google Scholar]
- 20.Schroy PC, 3rd, Heeren TC. Patient perceptions of stool-based DNA testing for colorectal cancer screening. Am J Prev Med 2005; 28: 208–214. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Hillman SL, Harris AM, et al. Patient perceptions of stool DNA testing for pan-digestive cancer screening: a survey questionnaire. World J Gastroenterol 2014; 20: 4972–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince M, Lester L, Chiniwala R, et al. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J Gastroenterol 2017; 23: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champion VL, Christy SM, Rakowski W, et al. A randomized trial to compare a tailored web-based intervention and tailored phone counseling to usual care for increasing colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2018; 27: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senore C, Basu P, Anttila A, et al. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut 2019; 68: 1232–1244. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Am J Gastroenterol 2017; 112: 1016–1030. [DOI] [PubMed] [Google Scholar]
- 26.American Cancer Society. Colorectal cancer prevention and early detection New York: American Cancer Society, 2016.
- 27.Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol 2016; 111: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gellad ZF, Stechuchak KM, Fisher DA, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol 2011; 106: 1125–1134. [DOI] [PubMed] [Google Scholar]
- 29.Cyhaniuk A, Coombes ME. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care 2016; 22: 105–111. [PubMed] [Google Scholar]
- 30.NHS. Bowel cancer screening. UK: NHS. [Google Scholar]
- 31.Environment NIfPHat. Bowel cancer screening programme, 2019. https://www.rivm.nl/en/bowel-cancer-screening-programme.
- 32.van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Adherence to colorectal cancer screening: four rounds of faecal immunochemical test-based screening. Br J Cancer 2017; 116: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph DA, King JB, Richards TB, et al. Use of colorectal cancer screening tests by state. Prev Chronic Dis 2018; 15: E80. [DOI] [PMC free article] [PubMed] [Google Scholar]