Abstract
The global prevalence of metabolic diseases such as type 2 diabetes mellitus, steatohepatitis, myocardial infarction, and stroke has increased dramatically over the past two decades. These obesity-fueled disorders result, in part, from the aberrant accumulation of harmful lipid metabolites in tissues not suited for lipid storage (e.g., the liver, vasculature, heart, and pancreatic beta-cells). Among the numerous lipid subtypes that accumulate, sphingolipids such as ceramides are particularly impactful, as they elicit the selective insulin resistance, dyslipidemia, and ultimately cell death that underlie nearly all metabolic disorders. This review summarizes recent findings on the regulatory pathways controlling ceramide production, the molecular mechanisms linking the lipids to these discrete pathogenic events, and exciting attempts to develop therapeutics to reduce ceramide levels to combat metabolic disease.
Keywords: obesity, insulin resistance, diabetes, ceramides, dyslipidemia, atherosclerosis
INTRODUCTION
The epidemic of obesity and its comorbidities is creating an enormous worldwide health burden, threatening the quality of life of families and the economic stability of countries around the globe. The number of individuals afflicted with obesity-related metabolic disorders—including diabetes, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH), and cardiovascular diseases (CVDs)—is astonishing. More than 450 million adults are living with diabetes (1); ~25% of the adult population has NAFLD/NASH (2); and ~30% of the population will die from cardiovascular disease (3). Studies published over the last two decades indicate that ceramides, which are intermediates in the biosynthetic pathway that produces complex sphingolipids, cause many of the cellular defects that drive these debilitating diseases. These discoveries about ceramides, described at length herein, present opportunities to understand the evolutionary pressures that lead to metabolic pathologies and to identify new therapeutic options for combating them.
Many of the early mechanistic studies probing the link between obesity, dyslipidemia, and cardiometabolic disease focused on insulin resistance, a condition where insulin is unable to effectively clear glucose from the bloodstream and a risk factor for all of the diseases listed above. Pioneering studies of Sir Philip Randle revealed that glucose and fat served as competitive substrates, with elevated fat effectively blocking utilization of carbohydrates (4). Randle and colleagues postulated a mechanism involving intermediates produced during lipid metabolism, which inhibit steps of glycolysis and thus might slow rates of glucose utilization. Subsequently, researchers found that complex lipids derived from excess fatty acids inhibited insulin signaling, thus blocking the postprandial translocation of the GLUT4 glucose transporters to the plasma membrane and concomitant glucose entry into muscle and fat tissue (5). Initially, the focus was on the glycerol-containing lipids (e.g., diacylglycerols and triacylglycerols), as their intramyocellular levels generally correlate with the severity of insulin resistance. However, sphingolipids such as ceramides were also found to accrue under conditions of lipid oversupply (6). The enigmatic ceramides were found to be potent inhibitors of insulin-stimulated GLUT4 redistribution, owing to their ability to block activation of Akt/PKB, a serine/threonine kinase that is a master inducer of many anabolic processes (7). One could restore insulin signaling and glucose uptake in lipid-treated myotubes and muscle fibers by using interventions that lowered ceramides, but not levels of the more abundant glycerol-containing lipids, such as the diacylglycerols (8-11).
Building upon these in vitro studies, researchers turned to rodent models to gauge the importance of ceramides as modulators of insulin sensitivity. In rat or mouse models of obesity or dyslipidemia, the administration of pharmacological reagents and/or genetic engineering strategies to lower ceramide levels restored insulin sensitivity owing to improvements in glucose utilization in skeletal muscle and adipose tissue, as well as suppression of gluconeogenesis in the liver (11). Remarkably, inhibition of ceramide production also reversed all of the other complications of obesity and insulin resistance including type 2 diabetes, NAFLD/NASH, atherosclerosis, hypertension, and cardiomyopathy (11-26). These findings indicated that ceramides likely had a much broader array of tissue actions, beyond those involving Akt/PKB and the inhibition of glucose disposal, that contribute to metabolic disorders.
Subsequent research identified numerous new ceramide actions that alter metabolism and compromise tissue function. They also revealed ceramides as strong biomarkers of insulin resistance, diabetes, and cardiovascular disease in large human cohorts, to the extent that they are now being measured clinically as an indicator of disease risk (27). Herein, we summarize these studies and speculate about the evolutionary pressures that gave ceramides their deleterious attributes. Moreover, we discuss possible therapeutic strategies to modulate ceramide levels and combat the spectrum of cardiometabolic diseases associated with obesity.
DISCOVERY OF SPHINGOLIPIDS
In 1884, German-born physician Johann L.W. Thudichum published a treatise describing the chemical constituents of the brain. Armed with only the rudimentary procedures available during his era, he determined that most lipids within the central nervous system comprised a glycerophosphate, fatty acids, and a base. Curiously, he also found a distinct, less-abundant lipid that contained no glycerol moiety. He named the unknown backbone sphingosine, a reference to the mythological Sphinx, which has a woman’s head attached to a lion’s body. Like their namesake, these enigmatic, sphingosine-containing lipids (i.e., sphingolipids) had a dichotomous structure, which included a polar head attached to an unknown hydrophobic body.
Ensuing technological developments enabled a precise determination of the structure of sphingosine, which distinguishes sphingolipids from other lipid classes (28, 29). Thanks to the work of the LIPID MAPS Lipidomics Gateway consortium (http://www.lipidmaps.org/), scientists now know the composition of thousands of distinct sphingolipid species. Researchers also characterized the enzymatic steps that control ceramide production and metabolism, cloning the majority of enzymes driving these reactions (30). The advent of these technologies provided new opportunities to probe the role of these molecules in biology, revealing their important roles in the development of disease.
Sphingolipids [e.g., ceramides, sphingomyelins, sphingosine, sphingosine-1-phosphate (S1P) and gangliosides] are far less abundant than glycerolipids, typically representing 2–15% of the total cellular lipidome. Despite being relatively minor components of membranes, they have potent biological activities, altering the physiochemical properties of lipid bilayers and regulating the activity of receptors and intracellular proteins (28). Ceramides, which are particularly low in abundance, have emerged as signals of lipid excess that initiate a host of cellular stress responses, leading ultimately to the induction of apoptosis (31). These actions have enormous relevance to cardiometabolic disease.
PATHWAYS CONTROLLING SPHINGOLIPID SYNTHESIS AND METABOLISM
Enzymatic pathways controlling the biosynthesis, degradation, and regeneration of ceramides dictate the composition of the cellular sphingolipidome.
De Novo Biosynthesis
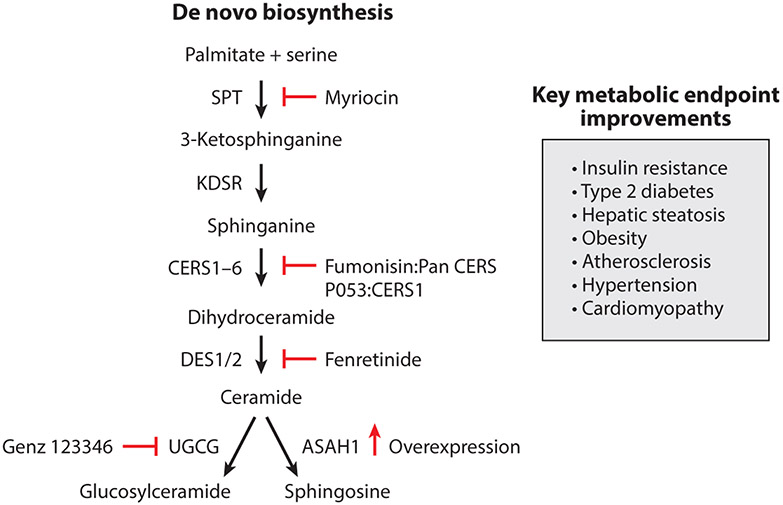
De novo biosynthesis of ceramides, the precursor of most complex sphingolipids, occurs on the cytosolic surface of the endoplasmic reticulum. The multistep enzymatic cascade starts with the condensation of palmitoyl-CoA (CoA) and serine to produce 3-ketosphinganine, a reaction that is catalyzed by serine palmitoyltransferase (SPT) (Figure 1). SPT comprises essential subunits encoded by the Sptlc1, 2, and 3 gene products, which form heterodimers with varying CoA preferences. For example, complexes containing SPTLC1 and 2 display a preference for C16-CoA, while complexes with SPTLC1 and 3 prefer C14-CoA (28, 29, 32). Additional, small subunits termed SSTPSA and SSTPSB enhance activity of the SPT heterodimers and confer additional specificity regarding fatty acid preference (33). Moreover, a family of small membrane-bound ORMDL proteins provide an addition level of regulation to SPT activity (34).
Figure 1.
De novo ceramide synthesis pathway and known inhibitors shown to improve metabolic diseases. Schematic of the de novo ceramide biosynthesis pathway highlighting the key enzymes involved in ceramide biosynthesis and their respective inhibitors. Abbreviations: ASAH1, acid ceramidase; CERS, ceramide synthase; DES, dihydroceramide desaturase; KDSR, 3-ketodihydrosphingosine reductase; SPT, serine palmitoyltransferase; UGCG, UDP-glucose ceramide glucosyltransferase.
The SPT complex can occasionally use alternative amino acids (i.e., alanine and glycine), instead of serine, to produce deoxysphingolipids. These rare sphingolipids were initially found in studies of individuals with hereditary sensory neuropathy, where mutations in SPT subunits alter its substrate selectivity (35). This finding prompted speculation that deoxysphingolipids elicited cellular damage, launching new studies of their relevance to disease processes, including many of the disorders that are the focus of this review (36-41).
In the canonical SPT reaction, the product 3-ketosphinganine is rapidly converted to sphinganine by 3-ketosphinganine reductase. This scaffold quickly acquires additional fatty acid side chains through n-acylation reactions catalyzed by a family of six (dihydro)ceramide synthases (CERS1–6). The CERS enzymes show variable tissue distribution patterns and distinct specificities for fatty acyl-CoA substrates. They produce a large number of dihydroceramides that include variable acyl chain lengths ranging from 14- to 34-carbon atoms. The complexity of the CERS family suggests that the acyl composition of sphingolipids influences cellular physiology in distinct, but still somewhat unresolved ways (42). Recent studies suggest that these CERS enzymes may also respond to fatty acid engagement by translocating to the nucleus, where they serve as transcriptional repressors of lipase genes (43).
The next reaction introduces a 4,5-trans-double bond into the sphingoid backbone of the dihydroceramides, producing the ceramides that are the major scaffold for most complex sphingolipids. This double bond, which is inserted by dihydroceramide desaturases (DEGS1 and 2 genes; DES1 and 2 proteins), bestows the unique biophysical properties that are instrumental for initiation of the stress responses that are the focus of this review (44). DES1 is a ubiquitous enzyme, while DES2 is an enzyme that can also produce phytoceramides and is enriched in the skin and intestinal epithelium (45, 46).
Once synthesized, ceramides are transported from the endoplasmic reticulum to the Golgi apparatus, where head groups are added to the oxygen residue at the first position to produce the complex sphingolipids (e.g., sphingomyelin, gangliosides, or ceramide 1-phosphate) (28, 32). At least one ceramide transporter protein (CERT1) has been identified, and this protein facilitates the passage of the ceramides to the Golgi apparatus for production of sphingomyelins (but not gangliosides). Ceramides can also acquire another acyl chain at this first position. Like their triglyceride counterparts in the parallel glycerolipid biosynthesis pathway, these tri-acylated ceramides can be stored in the lipid droplet (47). The reaction that produces 1-O-acylceramides uses fatty acyl-CoA esters generated by ASCL5, an acyl-CoA synthase, and ceramides as substrates. Diacylglycerol acyltransferase (DGAT)-2, an enzyme involved in the triglyceride synthesis (47), adds this acyl chain to ceramides, and thus plays roles in both the glycerolipid and sphingolipid synthesis pathways. In hepatocytes, channeling ceramide into 1-O-acylceramides inhibits apoptosis (47).
Ceramide Degradation
Ceramides are deacylated by ceramidases, which break them into their components: a variable free fatty acid and sphingosine (48). Until recently, the family of mammalian ceramidases included only five enzymes, classified by their pH optima [i.e., acid (ASAH1), neutral (ASAH2), and alkaline (ACER1–3) ceramidases]. These enzymes display different cellular locations and substrate preference (48). Interestingly, Scherer and colleagues found that adiponectin receptors (ADIPOR1 and 2) also have ceramidase activity (49, 50). Adiponectin is a cardioprotective and antidiabetic protein that is released from healthy adipose tissue and circulates at high concentrations. Upon ligand binding, the receptors undergo a conformational shift that enhances ceramidase activity. Substitution of inactivating residues in the ceramidase motif renders the receptor ineffectual (49). Granier and colleagues (51) purified the receptor in the presence of ceramide, determining that the resolved crystal structure contained a bound fatty acid located in a hydrophobic pocket; this region resembles similar structural domains found in the other ceramidases. This group’s studies with the purified protein confirmed that it had ceramidase activity (51).
Sphingosine can be phosphorylated to produce S1P, a bioactive lipid that gets secreted from the cell and activates a family of G protein–coupled receptors. S1P elicits biological responses that often oppose those of ceramide. S1P can be degraded by either sphingosine phosphate phosphatase (SPP) or S1P lyase. SPP dephosphorylates S1P to sphingosine, allowing for the reformation of ceramide through the salvage pathway discussed below. S1P lyase irreversibly converts S1P to ethanolamine phosphate and hexadecanal, thus breaking the sphingoid backbone and enabling an exit from the sphingolipid pathway (52).
Ceramide Regeneration
Ceramides can also be reproduced by the catabolism of sphingomyelin (e.g., through the action of sphingomyelinases), which generates ceramides while liberating the choline head group (53, 54). This reaction is catalyzed by a family of sphingomyelinases that differ by subcellular location and pH optimum. Alternatively, the sphingosine generated by ceramidases can also be reacylated by CERS enzymes to regenerate ceramides (28, 32, 52). This reacylation process is termed the salvage pathway. Glycosphingolipids can also be hydrolyzed by glycosidases, but this step is less frequent and accounts for a smaller fraction of ceramides than the aforementioned sphingomyelinase and ceramidase reactions (55, 56).
CLINICAL RELATIONSHIPS BETWEEN SERUM OR TISSUE SPHINGOLIPIDS AND CARDIOMETABOLIC DISEASES
Advances in mass spectrometry and lipidomics have enabled sensitive and accurate characterization of the sphingolipidome in human serum and tissue samples. These studies have revealed strong relationships between circulating sphingolipids and insulin resistance (57, 58), diabetes (59, 60), CVD incidence (61), secondary CVD events (62), secondary CVD mortality (63-65), and coronary artery disease (66). Because of the strength of these relationships, which often surpass those of other lipid biomarkers (e.g., LDL cholesterol), clinics in the United States and Europe now offer diagnostic tests to measure circulating ceramides as measures of CVD risk (27). Herein, we detail these clinical studies and discuss the potential of using serum sphingolipids for disease diagnosis and patient stratification.
Ceramides as Markers of Insulin Resistance and Diabetes
Large studies in Australians, Chinese Singaporeans, and Native Americans, some involving sample numbers in the thousands, have revealed strong associations between circulating ceramides, insulin resistance, and type 2 diabetes. Meikle and colleagues (67) profiled 640 individuals enrolled in the Australian Diabetes, Obesity and Lifestyle Study, finding that ceramides positively correlated with fasting blood glucose. Herr and colleagues (57, 58) profiled 2,302 ethnically Chinese Singaporeans, finding a positive relationship between most ceramide species and homeostatic model of insulin resistance (HOMA-IR) scores. In addition, Lemaitre et al. (58) profiled 2,086 Native Americans, finding that several ceramide species (e.g., C16:0 and C18:0) correlated with HOMA-IR scores. In a nutritional interventional study, Yki-Jävinen and colleagues (68) also found that overfeeding saturated fats increased serum ceramides in plasma while producing a concomitant decline in insulin sensitivity.
Longitudinal studies indicate that serum sphingolipid levels increase early in disease progression, and thus might serve as predictive biomarkers of future disease. For example, Thorens and colleagues (60) found that dihydroceramides, which are the less-abundant precursors of ceramides, were elevated in individuals up to 9 years prior to occurrence of type 2 diabetes. These relationships held true in two independent clinical cohorts. In general, we have found that the dihydroceramides, owing to their low abundance, change more dynamically than ceramides under conditions of increased flux both in vitro and in vivo (S.A. Summers, unpublished observations).
Studies evaluating tissue levels of ceramides also reveal positive associations between ceramides and insulin resistance. In liver, Yki-Jävinen and colleagues (69) analyzed 125 liver biopsies obtained from individuals of Finnish descent, finding that C16:0, C18:0, C20:0, C22:0, and C24:1 ceramide species correlated with insulin resistance. These relationships were independent of hepatic triglycerides and body weight.
In adipose tissue, Yki-Jävinen’s team (70) similarly found that ceramides correlated with insulin resistance, independent of obesity. Moreover, Brüning and colleagues (26) profiled 439 individuals of European descent, finding numerous ceramide species that were elevated in the adipose tissues of obese individuals. His group also demonstrated that adipose CERS6 transcripts correlated positively with body mass index (BMI), body fat content, and hyperglycemia (26). By comparison, CERS6 negatively correlated with glucose infusion rates during euglycemic-hyperinsulinemic clamps (26). These data support rodent studies (discussed below) revealing that C16:0 ceramides derived from CERS6 antagonize insulin action.
In skeletal muscle, Goodpaster and colleagues (71-74) identified positive correlations between muscle ceramides and insulin resistance in a large number of separate studies. In these studies, insulin-sensitizing interventions (e.g., exercise, metformin, pioglitazone, and bariatric surgery) decreased muscle ceramide levels (71-74). Mandarino and colleagues (75) reported similar findings in muscle biopsies.
Ceramides as Biomarkers of Cardiovascular Disease
A particularly large number of studies, many conducted by Laaksonen and colleagues at Zora Biosciences, have identified serum ceramide species that are potent, independent, and predictive markers of coronary artery disease, major adverse cardiac events, and/or fatality (58, 63, 65, 76-84). They also determined that plasma ceramides predict recurrent heart attacks and cardiovascular-related deaths in patients that had undergone a prior cardiac event (62). Moreover, they found that statins decrease plasma ceramides (84, 85). In each of these studies, three ceramide species (C16:0, C18:0, and C24:1) reliably predicted the severity of cardiovascular disease. Using these data, the authors generated a ceramide-based score (i.e., CERT1) that included these species in proportion to C24:0, a benign ceramide that shows no relationship with disease and could thus be used for normalization (86). CERT1 predicted coronary death more than three times better than LDL cholesterol. Clinics are now offering CERT1 diagnostic tests (27). More recently, this group improved upon CERT1, producing a refined CERT2 score that included additional markers and showed enhanced prognostic utility (78).
Our group also determined that sphingolipids were strong, cholesterol-independent biomarkers of coronary artery disease (66). In this study, 30 different sphingolipids demarcated individuals with the disease. Using machine learning, we determined that several low-abundance sphingolipids, which are likely good markers of flux through the biosynthesis pathway, were particularly strong markers. Using this information, we created a Sphingolipid Inclusive Score (SIC), which proved superior to CERT1 and LDL cholesterol for that patient population.
In tissues, Schulze and colleagues found elevated myocardial ceramides in patients with advanced heart failure (18, 87).
Efficacy of Ceramide-Reduction Interventions in Rodents
Many of the interventional studies exploring the relevance of sphingolipids to cardiometabolic disorders were conducted with myriocin, an irreversible and high-affinity inhibitor of SPT. In rodents, chronic treatment with myriocin has been shown to prevent and/or reverse atherosclerosis (15-17, 22-24), insulin resistance (11, 88-91), diabetes (11), NAFLD/NASH (92), hypertension (90), and cardiomyopathy (18, 21). The astonishing utility of this single compound in such a large array of disease models has generated considerable excitement about the potential of therapeutics designed to lower ceramides. Subsequent studies, discussed below, confirmed the importance of ceramides in cardiometabolic disorders using genetic engineering strategies to inhibit rates of ceramide synthesis or accelerate rates of ceramide degradation. This research, in addition to revealing the involvement of sphingolipids in pathology, has enabled determination of tissue-specific roles of ceramides as drivers of tissue dysfunction.
Ceramide-lowering interventions resolve insulin resistance and prevent diabetes.
Myriocin was shown to prevent and/or reverse insulin resistance in high-fat diet-fed mice (11, 25, 89, 91), lipid-infused rats (11), fructose-fed hamsters (93), and leptin-deficient mice and rats (i.e., Zucker fa/fa rats and ob/ob mice) (11). Moreover, myriocin showed efficacy in models where the insulin resistance progresses to frank diabetes. Specifically, Zucker diabetic fatty rats transform from a prediabetic state characterized by insulin resistance and hyperinsulinemia to frank diabetes that includes fasting hyperglycemia, decreased insulin, and declining beta-cell mass. Myriocin protects these animals from the progression of beta-cell failure and the concomitant development of diabetes (11). Unger and colleagues (94) also found that cycloserine, a broad-spectrum antibiotic that also inhibits SPT, prevents beta-cell failure in Zucker diabetic fatty rats.
In mice fed a high-fat diet, the insulin-sensitizing effects of myriocin could be recapitulated with fenretinide (95, 96), a synthetic retinoid that inhibits DES1 (96). The beneficial actions of fenretinide were initially attributed to its effects on retinol-binding protein 4 (RBP4), which has also been implicated in the development of insulin resistance. However, fenretinide shows efficacy in RBP4-knockout mice (97), suggesting that the compound has other insulin-sensitizing mechanisms. Indeed, it dramatically lowered ceramide levels (and increased dihydroceramides) in concert with its inhibition of DES1 and improvement in metabolic homeostasis (96).
Additional studies reveal that removal of genes required for ceramide biosynthesis or overexpression of those that catalyze ceramide degradation is also insulin sensitizing. For example, we found that inducible depletion of Degs1 in the entire periphery, as well as tissue-specific removal from the liver, adipose tissue, or the liver plus adipose tissue, was sufficient to restore insulin sensitivity in high-fat diet-fed and/or leptin-deficient ob/ob mice (12). The following interventions also improved insulin sensitivity in mice fed a high-fat diet: (a) adipose-specific depletion of a gene encoding a key subunit of SPT (i.e., Sptlc2) (88), (b) whole-body or brown adipose tissue–specific depletion of Cers6 (26, 98), or (c) whole-body or muscle-specific depletion of Cers1 (99). Scherer and colleagues (100) also found that overexpression of acid ceramidase (Asah1), which lowers ceramides by inducing their conversion to sphingosine, in either adipose tissue or the liver, improved insulin sensitivity in high-fat diet-fed mice.
Ceramide-lowering interventions resolve dyslipidemia and NAFLD/NASH.
Most of the models described in the preceding section also evaluated liver and serum lipid levels, revealing that ceramide-lowering interventions led to improvements in lipid homeostasis. For example, treatment with myriocin (11, 20, 25) or fenretinide (96), as well as depletion of Sptlc2 (adipose) (88), Cers6 (whole body or brown adipose tissue) (26, 98), or Degs1 (whole body, adipose tissue, or liver) (12), resolved hepatic steatosis and/or reduced circulating triglycerides. Liver- or adipose-specific overexpression of Asah1 also resolved steatosis and dyslipidemia. Recently, Jiang et al. (92) tested myriocin in a rat model that progresses from hepatic steatosis (NAFLD) to NASH, characterized by pronounced liver fibrosis. Myriocin prevented the progression of fibrosis that defines NASH.
Ceramide-lowering interventions resolve atherosclerosis.
Myriocin also prevents development of atherosclerotic lesion formation in apolipoprotein E (ApoE) knockout mice (17, 22, 24). In some instances, the intervention was also shown to enable regression of preformed plaques. Genetically, depletion of Sptlc2 from myeloid cells was sufficient to reduce the atherosclerotic plaque area in LDL-receptor knockout mice (101).
Ceramide-lowering interventions ameliorate vascular dysfunction.
In mice fed a high-fat diet, myriocin and haploinsufficiency for Degs1 resolved hypertension (90, 102). The effects could be recapitulated in isolated blood vessels exposed to high levels of saturated fat, with the studies indicating that these ceramide actions likely occurred in the endothelial cell.
Ceramide-lowering interventions ameliorate cardiomyopathy and heart failure.
Ceramide-reducing strategies, including either myriocin or haploinsufficiency for Sptlc2, have also shown to correct hypertrophy and improve cardiac function in the following mouse models of heart disease: (a) lipotoxic cardiomyopathy (i.e., overexpression of lipoprotein lipase in cardiomyocytes) (21) and (b) coronary occlusion (18, 103). Russo et al. (104) identified lipids produced by CERS5 as possible mediators of cardiac hypertrophy.
Fundamental Cellular Mechanisms Linking Ceramides to Cardiometabolic Disease
Studies conducted in mice that involve whole-body or tissue-specific manipulations of SPT (e.g., the SPTLC2 subunit), DES1, ASAH1, or the various CERS isoforms have revealed important information about the mechanisms linking ceramides to the tissue dysfunction that drives cardiometabolic disease. Complementary studies in cell culture systems have further illuminated downstream pathways and intracellular mediators of these ceramide actions. In this section, we discuss the data (a) resolving which ceramides are lipotoxic and (b) elucidating fundamental ceramide actions that are relevant to disease states.
Identification of Roles for C16:0 and C18:0 Ceramides as Key Lipotoxic Species in Adipose Tissue, Skeletal Muscle, and/or the Liver
Though numerous different sphingolipid species likely contribute to the decline in tissue dysfunction in obesity and cardiometabolic disease, ceramides containing the C16 and C18 acyl chains (C16:0 and C18:0) have proven to be particularly damaging. By contrast, those containing much longer chains (e.g., C24 or C24:1) appear to be either protective or benign.
In 2014, Brüning and colleagues (26) provided the strongest evidence to date that C16:0 ceramides drove the decline in adipose and liver function in obesity and the concomitant disruption in glucose and lipid homeostasis. They generated knockout mice allowing for whole-body or tissue-specific (i.e., liver or brown adipose tissue) ablation of the gene (Cers6) encoding CERS6, the enzyme that produces C16:0 ceramides. Depleting Cers6 from any of these locales resolved high-fat diet–induced obesity, glucose tolerance, and insulin resistance in mice (26). They later determined that these improvements were due, in part, to a reduction in mitochondrial fission (discussed below) (98). In parallel, our laboratory found that mice that were haploinsufficient for the Cers2 gene that encodes CERS2, which produces the C20–C24 ceramide species, demonstrated a worsening of glucose tolerance and hepatic steatosis (25). This surprising result became understandable when we observed a large, compensatory induction of the CERS6 isoform and a concomitant increase in C16:0 ceramides. Moreover, we found that overexpression of CERS6, but not CERS2, induced fat accumulation and antagonized insulin action in primary hepatocytes. These complementary papers, published by the Brüning (26) and Summers (25) laboratories in the same issue of Cell Metabolism, revealed remarkably similar mechanisms involving C16:0 ceramide actions on mitochondrial efficiency and insulin signaling. Shortly thereafter, researchers at Sanofi used an antisense oligonucleotide approach to deplete CERS6 from mice. In this study, Tennagels and colleagues (105) demonstrated that inhibition of CERS6 reduced C16:0 ceramides and fat content and improved glucose tolerance and insulin sensitivity. Hoch and colleagues (106) obtained similar results in mice lacking CERS5, another enzyme that produces C16:0 ceramides.
Brüning and colleagues (99) also studied mice lacking CERS1, the enzyme that produces C18:0 ceramides and the major ceramide synthase isoform in skeletal muscle. They determined that depletion of the gene globally (i.e., in all tissues) or selectively in skeletal muscle conferred protection from obesity-induced insulin resistance. Don and colleagues (107) also synthesized a selective inhibitor of CERS1, termed P053. Chronic administration of P053 to obese mice impeded fat deposition, though it had unimpressive effects on insulin resistance (107). Mechanistically, these actions of CERS1 were attributed to effects on fatty acid metabolism in the skeletal muscle and increased secretion of fibroblast growth factor 21 (99, 107). Additional studies are necessary to better clarify the importance of C18:0 ceramides in cardiometabolic disease.
Several studies conducted either in vivo or in vitro evaluated the relevance of the ceramide headgroups in insulin resistance and metabolic dysfunction. Deletion of sphingomyelin synthase 1 from mice was shown to be insulin sensitizing, but the animals were resistant to weight gain (108); the disparity in body mass thus made it difficult to discern whether sphingomyelin is a direct regulator of insulin sensitivity. Studies conducted in vitro suggest that ceramides, rather than the more abundant sphingomyelins, were the key modulators of metabolism. Inhibition of sphingomyelin synthase isoforms induced ceramide accumulation by blocking its conversion into sphingomyelin (109). This intervention elicited many of the downstream mechanisms described below (i.e., inhibition of insulin signaling and impaired mitochondrial respiration) (109).
Additional studies evaluated the role of glucosylated ceramides, including the gangliosides, as drivers of metabolic dysfunction. Inhibitors of glucosylceramide synthase have shown efficacy in models of steatosis and insulin resistance (110-112), with mixed results seen in models of atherosclerosis (113-115). Moreover, knockout of GM3 synthase, which produces higher-order gangliosides, is also insulin sensitizing in vitro and in vivo (116-118). These data indicate that glucosylated ceramides also contribute to cardiometabolic disease, in part by inhibiting insulin signaling. Our own studies revealed that ceramides and glucosylceramides have separable and independent actions on insulin signaling, depending on the tissue type being studied (119).
Ceramide Regulation of Mitochondrial Biology (i.e., Energetics and Apoptosis)
Ceramides exhibit numerous actions on mitochondria, leading to diminished electron transport chain activity and increased mitochondrial outer membrane permeability. These actions lead to decreased mitochondrial respiratory capacity and increased tissue apoptosis, respectively. Both of these are hallmarks of diabetes and cardiovascular disease.
Ceramides have been shown to decrease mitochondrial respiration by inhibiting oxidative phosphorylation and promoting mitochondrial fragmentation, leading to an induction of reactive oxygen species (25, 26, 98) (Figure 2). Treating cells or isolated mitochondria with ceramide analogs acutely disrupts components of the electron transport chain (120-122). Similar findings were obtained after overexpressing CERS6, which produced mitochondrial C16:0 ceramides and inhibited complex II (25). Moreover, depletion of sphingomyelinase synthase 2 produced impairments in mitochondrial respiration in association with an elevation in intracellular ceramides (109). Lastly, knockout of the genes encoding SPTLC2, CERT1, CERS6, CERS2, and DEGS1 leads to changes in mitochondrial energetics that inversely mirror tissue ceramide levels (12, 25, 26, 88, 98, 99, 107).
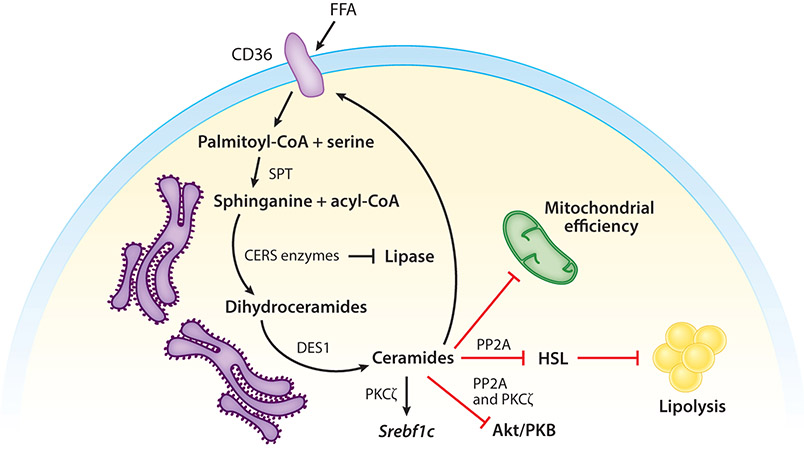
Figure 2.
Molecular mechanisms via which ceramides gauge intracellular lipids. Ceramides regulate metabolism through the following mechanisms: translocation of CD36 toward the plasma membrane, thus facilitating the safe uptake of FFA and enhancing the conversion of fatty acids into acyl-CoA, induction of Srebf1, inhibition of Akt/PKB, inhibition of HSL, safe storage of ceramide as 1-O-acylceramide inhibition of mitochondrial metabolic efficiency, and inhibition of lipases. Abbreviations: Akt/PKB, protein kinase B; CD36, cluster of differentiation 36; CERS, ceramide synthase; DES1, dihydroceramide desaturase 1; FFA, free fatty acid; HSL, hormone-sensitive lipase; PKCζ, protein kinase zeta; PP2A, protein phosphatase 2A; SPT, serine palmitoyl transferase; Srebf1, sterol regulatory element-binding protein 1.
Hammerschmidt et al. (98) determined that ceramides were potent regulators of mitochondrial fission. Using a proteomics approach to detect proteins that interacted with sphingolipids produced by CERS6, they determined that mitochondrial fission factor (MFF) was a ceramide target. The protein linked ceramides to an acceleration of mitochondrial fission, which resulted in a change in mitochondrial morphology and a decrease in respiratory capacity (98). Moreover, they found that CERS6-knockout mice contained numerous large, highly efficient mitochondria (98). While these actions do not explain the acute effects of ceramides on mitochondrial respiration, they do provide one mechanism by which chronic increases in ceramides disrupt mitochondrial respiration.
As ceramide levels continue to rise, they increase permeability of the mitochondrial outer membrane. This leads to the release of cytochrome c and initiation of apoptosis (123). Ceramides promote translocation of the proapoptotic Bcl2 family proteins (e.g., BAX) to mitochondrial membranes (124). They also bind voltage-dependent anion channel 2 (VDAC2), which further increases mitochondrial membrane permeability (125). Lastly, C16:0 ceramides form channels in mitochondrial membranes that can allow passage of cytochrome c (126-129).
Ceramides Inhibit Insulin Signaling to Block Glucose Uptake and Storage
Ceramides inhibit insulin and growth factor action by preventing activation of Akt/PKB, a serine/threonine kinase that stimulates glucose utilization, promotes cell survival, and activates enzymes driving fatty acid, protein, and triglyceride synthesis. Ceramides influence Akt/PKB via two distinguishable actions on distinct kinase domains. First, they accelerate the dephosphorylation of two important Akt/PKB residues on Akt/PKB via protein phosphatase 2A (PP2A) (9, 130-132). The action of ceramides on PP2A may be direct (133), but they also regulate the enzyme by displacing the PP2A inhibitory protein I2PP2A (102). In certain cell types, the inhibitory actions of ceramides on Akt/PKB could be negated either by treating with PP2A inhibitors (e.g., okadaic acid) or by overexpressing the SV40 small T antigen (which blocks access of PP2A to its substrates) (8, 9, 130-132). Second, ceramides have also been shown to block Akt/PKB translocation to the membrane by stimulating its phosphorylation on a separable, inhibitory residue in the pleckstrin homology domain (134, 135). This mechanism requires a ceramide-driven association of protein kinase Cζ (PKCζ) with caveolar domains (136). Both of these pathways can exist within the same cell type (137). In insulin-responsive tissues, the ceramide actions on Akt/PKB account for the impairments in insulin-stimulated GLUT4 glucose transporter translocation to the plasma membrane (7).
Ceramides Stimulate Lipid Uptake and Storage
Within the last few years, ceramides were found to elicit a new spectrum of actions that altered lipid handling in adipocytes and hepatocytes. First, they facilitate the uptake and esterification of fatty acids by promoting the redistribution of fatty acid translocases like CD36 (12, 49). Second, they induce genes (e.g., SREBP) that promote the incorporation of free fatty acid into triglycerides to facilitate their storage in lipid droplets (12, 138). Third, they inhibit lipolysis by blocking activation of hormone-sensitive lipase (HSL) (12). These effects are also attributable to the aforementioned ceramide effects on PKCζ and PP2A (12, 49, 138).
TISSUE-SPECIFIC ROLES OF CERAMIDES IN CARDIOMETABOLIC DISEASE
Tissue-specific interventions revealed how the fundamental ceramide actions described above manifest themselves in mammals. For a comprehensive list of transgenic mice used to discern the role of ceramide and ceramide metabolites in metabolic diseases, see Table 1.
Table 1.
Summary of mouse models used for manipulating de novo ceramide synthesis pathway in vivo to determine their role in metabolic diseases
| Gene | Knockout/overexpression | Phenotype |
|---|---|---|
| Sptlc1 | Whole body | Embryonic lethal (201) |
| Adipose tissue | Lipodystrophy (139) | |
| Sptlc2 | Whole body | Embryonic lethal (201, 202) |
| Haploinsufficiency | Improve insulin sensitivity and resolve hepatic steatosis (108) | |
| Liver | ||
| Adipose tissue | Increase energy expenditure, improve insulin sensitivity, and resolve hepatic steatosis (88), lipodystrophy (140) | |
| Myeloid cells | No phenotype (88) | |
| Intestinal cells | Lethal (203) | |
| CerS1 | Whole body | Increase energy expenditure, improve insulin sensitivity (99) |
| Skeletal muscle | ||
| CerS2 | Haploinsufficiency | Impair insulin sensitivity and develop hepatic steatosis (25) |
| CerS3 | Whole body | Impair skin barrier function, lethal (204) |
| CerS4 | Whole body | Alopecia (205) |
| CerS5 | Whole body | Two studies: one demonstrating improved insulin sensitivity (106) and another showing no phenotype (26) |
| Skeletal muscle | No phenotype (99) | |
| CerS6 | Whole body, liver, brown adipose tissue | Increase energy expenditure, improve insulin sensitivity, and resolve hepatic steatosis (26) |
| Myeloid cells | No phenotype (26) | |
| Skeletal muscle | No phenotype (99) | |
| Degs1 | Whole body, liver, adipose tissue | Improve insulin sensitivity and resolve hepatic steatosis (12) |
| Myeloid cells | No phenotype (12) | |
| Intestinal cells | No phenotype (12) | |
| Asah1 | Liver (overexpression) Adipose tissue (overexpression) | Improve insulin sensitivity and resolve hepatic steatosis (100) |
| Ugcg | Intestinal cells | Regulate nutrient uptake (206) |
| Central nervous system and hypothalamus | Regulate energy homeostasis and peripheral lipolysis (167, 168) |
Ceramides in the Adipocyte
In mice and rats, myriocin decreases adipocyte size, elevates numbers of brown/beige adipocytes, and increases recruitment of M2 macrophages into the subcutaneous adipose bed (88). These effects were most robust in the subcutaneous depots. Similar results were obtained following adipocyte-specific depletion of Sptlc2, which induced a comparable spectrum of benefits and was sufficient to improve insulin sensitivity and glucose tolerance, resolve hepatic steatosis, increase whole-body energy expenditure, and enhance adipocyte respiration (88).
We have conducted analogous studies by depleting Degs1 from adipocytes (12). The absence of the DES1 protein reduced adipocyte size, increased glucose uptake into the adipose tissue, and enhanced mitochondrial respiration. These effects were strong enough to improve glucose tolerance and insulin sensitivity and resolve hepatic steatosis. Similar findings were obtained following brown adipose tissue–specific CerS6 depletion (26) or adipose-specific Asah1 overexpression (100). Because of the comparable phenotypes observed in all of these studies, we conclude that ceramides act as nutrient signals that direct the adipocyte toward a hypometabolic phenotype.
We note one important distinction between the studies involving SPT inhibition or Sptlc2 depletion and those involving manipulation of other enzymes in the pathway. Unlike inhibition of SPT, neither removal of Degs1 nor overexpression of Asah1 induced browning/beiging of the adipose depots. We speculate that the discordance may be attributable to intermediates in the biosynthetic pathway, which are downstream of SPT and upstream of DES1. These intermediates (e.g., the CERS enzymes, which can act as transcriptional repressors) may influence the browning program.
Notably, studies by the Proia (139) and Park (140) laboratories found that ablation of either Sptlc1 or 2, respectively, in adipose tissue impaired adipose differentiation and elicited lipodystrophy. In these studies, the authors used an adiponectin-Cre-recombinase mouse (141) that expressed the transgene early in development (142). By comparison, the adiponectin-Cre mouse we used, from the Scherer laboratory, used a different promoter fragment that expresses the recombinase weaker and later (88). In concordance with their work, our studies in primary cells showed that inhibition of SPT inhibits adipocyte differentiation in vitro (88).
Ceramides in the Hepatocyte
Treating mice with either myriocin or fenretinide reduces hepatic lipid accumulation and represses hepatic gluconeogenesis (11, 96). Tissue-specific ablation of DES1, either by genetic knockout or knockdown using short hairpins, from the liver of mice conferred comparable metabolic benefits (12). These findings, and complementary data from researchers studying cultured hepatocytes, confirm that ceramides have cell-autonomous actions within the hepatocyte. Mechanistically, these effects result from ceramide’s ability to (a) induce triglyceride synthesis by modulating Srebf1 expression, (b) promote fatty acid uptake by altering CD36’s subcellular distribution, and (c) enhance gluconeogenesis by blocking AKT/PKB phosphorylation (12). These actions of ceramides lead to increased fat deposition and glucose output, thus producing the “selective insulin resistance” (143) that defines the prediabetic condition.
The Scherer group (100) previously conducted an elegant study that elucidated many of these mechanistic details. Their group found that liver-specific overexpression of acid ceramidase reduced hepatic ceramide content, improved insulin sensitivity, and resolved hepatic steatosis (100). This study was the first to show that ceramide induced translocation of CD36 via PKCζ (100).
Brüning and colleagues (26, 98) also performed liver-specific interventions with CERS6, finding that its removal from the liver protected mice from steatosis and insulin resistance. A major mechanism was related to the aforementioned improvements in mitochondrial function. We similarly found that overexpressing CERS6 in hepatocytes induced triglyceride production, inhibited insulin signaling, and induced mitochondrial dysfunction (25).
Ceramide Actions in the Myocyte
The question of whether ceramides modulate metabolism in skeletal muscle has been an area of debate. The disagreement is largely due to a discordance in lipidomic profiling studies. Though many researchers found correlations between muscle ceramides and insulin sensitivity (described above), a small subset did not (144, 145). Despite this contention, interventional studies unequivocally support roles for ceramides as modulators of insulin signaling and mitochondrial function in rodents and cultured myotubes. In cellular models, ceramides invariably and potently inhibit insulin signaling and decrease mitochondrial respiration (9, 109, 146, 147). Moreover, in myotubes or rodent muscle fibers, blocking ceramide synthesis or accelerating ceramide degradation attenuates palmitate- or TLR4-induced insulin resistance (i.e., inhibition of insulin signaling and/or glucose uptake) (8, 9, 11, 119, 135, 147). In rodents, pharmacological inhibition of SPT or DES1, as well as depletion of Degs1, improved muscle insulin sensitivity in mice (11). Such experiments have been conducted in mice or rats fed a high-fat diet, treated with dexamethasone, intravenously infused with lipid cocktails, or lacking leptin (11).
These data indicate that ceramides produced within myocytes can operate as cell-autonomous regulators of metabolism. Nonetheless, Watt and colleagues (148) have presented the provocative idea that circulating ceramides may be even more important as determinants of muscle insulin sensitivity. His group found that ceramides present in LDLs were sufficient to induce insulin resistance (148). Lipoprotein particles lacking ceramides were incapable of antagonizing insulin action in muscle, but those containing the sphingolipid had potent effects. This finding suggests that lipoprotein-bound ceramides may be important drivers of muscle insulin resistance.
Ceramide Action in the Pancreatic Beta-Cell
Myriocin preserves pancreatic beta-cell mass in Zucker diabetic fatty rats (11). While this could simply be secondary to improved insulin sensitivity, which would alleviate the burden on the beta-cell, several in vitro studies suggest that ceramides might have cell-autonomous actions within the islet. Indeed, the earliest studies relating ceramides to diabetes were conducted by Roger Unger’s laboratory, which found that ceramides were responsible for palmitate-induced beta-cell apoptosis (94, 149). Subsequent studies also implicated ceramides in the impairment of insulin secretion and insulin gene transcription (94, 150-157). Glycosylated derivatives of ceramide (i.e., gangliosides) have also been identified as putative antigens that contribute to the auto-immune response (158-161). These in vitro studies suggest that ceramides could contribute to the decline in beta-cell function that underlies diabetes. Nonetheless, the critical animal experiments allowing for beta-cell-specific modulation of ceramides in vivo have not been conducted.
Ceramides in Cardiomyocytes and the Vascular Endothelium
Myriocin prevents or reverses numerous cardiovascular conditions (e.g., atherosclerosis, hypertension, cardiomyopathy, and heart failure) in rodents (22-24, 102, 162). Though these effects could be a consequence of improvements in insulin sensitivity and dyslipidemia, additional studies suggest that ceramides have deleterious actions in cardiac and vascular tissues. For example, the atherogenic effects of ceramide may result from direct actions in blood vessel walls, as they induce transcytosis of oxidized low-density lipoproteins. This action leads to lipid retention (163) and monocyte adhesion (164), thus driving plaque formation. In addition, ceramides may alter vascular reactivity. Studies in isolated blood vessels indicate that ceramides induced colocalization of PP2A with eNOS, leading to the inhibition of eNOS phosphorylation and its dissociation from Akt/PKB (90). Lastly, ceramides may directly compromise cardiomyocyte function, as pharmacological or genetic inhibition of ceramide synthesis in a model of lipotoxic cardiomyopathy (i.e., cardiac-specific overexpression of human lipoprotein lipase) abrogates apoptosis and cardiac contraction (24). Unfortunately, heart-specific deletion of Sptlc2 impairs cardiac function (140), making it difficult to ascertain whether the ceramides that regulate cardio-lipotoxicity are generated within the cardiomyocyte.
Ceramides in the Hypothalamus
Ceramides accumulate in the hypothalamus of mice following either high-fat diet feeding or acute lipid infusion (89). They are presumed to serve as nutritional signals that modulate appetite or metabolic rate. Experimental delivery of ceramides into the hypothalamus promotes weight gain and reduces brown adipose tissue thermogenesis (165). By contrast, intracerebroventricular infusion of myriocin reduces ceramide accumulation and improves hypothalamic insulin sensitivity, improving glucose tolerance and restoring beta-cell mass (166). Additional studies suggest that glycosylated ceramides may serve as important modulators of central nervous system function. Mice deficient in glycosphingolipids in the hypothalamus develop progressive obesity due to decreased thermogenesis (167). Moreover, adeno-associated virus (AAV)-mediated Ugcg (encoding for GCS) delivery to the hypothalamic arcuate nucleus led to ensuing elevations in nuclear glucosylceramides and subsequent weight loss. Mechanistically, GCS-depleted neurons displayed inadequate leptin receptor activation, requiring neuronal gangliosides GM1 and GD1a to be recruited to the leptin receptor upon ligand stimulation (167). In a follow-up study, Nordström and colleagues (168) ablated Ugcg in the neurons that target the mediobasal hypothalamus. They found that glucosylceramides regulate fasting-induced lipolysis by regulating norepinephrine content in the adipose depots. These studies raise the possibility that products of the sphingolipid pathway serve as important gauges of fuel load in the central nervous system.
REGULATION OF CERAMIDE SYNTHESIS AND METABOLISM
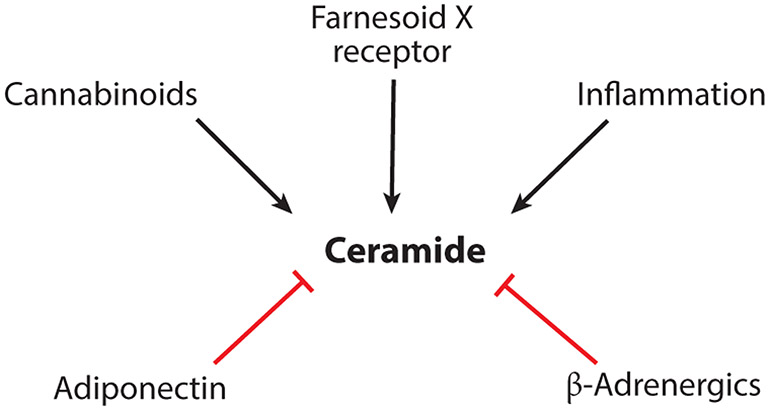
The ceramide biosynthesis pathway serves as a gauge of nutritional excess that reflects the supply of precursor substrates such as palmitoyl-CoA and serine (169). In human studies, diets enriched in saturated fat, but not unsaturated fat, increase ceramide accumulation (68). Similar findings are reported in rodents, with C16:0 ceramides emerging in unbiased metabolomic studies across multiple studies and strains as good markers of diet-induced metabolic pathologies (170). Beyond nutritional load, however, researchers have determined that several regulatory factors control rates of ceramide synthesis and degradation (Figure 3).
Figure 3.
Key regulators of ceramide accumulation. Depicted are the key regulators of ceramide synthesis thus far identified and involved in metabolic diseases.
Inflammation
Obesity is associated with increased systemic inflammation, including a particularly robust recruitment and activation of macrophages within the expanded adipose depots (171). The resulting increase in inflammatory cytokines in serum and tissues enhances rates of ceramide synthesis. Indeed, tumor necrosis factor (TNF)-α, interleukins, and chemokines selectively increase levels of sphingolipids, typically without affecting glycerolipids (172). The combination of increased nutrient supply and inflammation in obesity thus leads to synergistic enhancement of ceramide production (173). Indeed, inflammatory cytokines show a particularly tight association with ceramides in subjects with insulin resistance (172, 174).
One of the key modulators of ceramide biosynthesis is Toll-like receptor-4 (TLR4), a pattern recognition receptor that activates NF-κB and drives innate immunity. A particularly well-known receptor agonist is lipopolysaccharide, but the receptor also binds to low-density lipoprotein, viral proteins, polysaccharide, and several other ligands. One of the more intriguing findings relates to saturated fatty acids, which were initially identified as putative ligands (175) but were later found to amplify receptor signaling (176). The presence of an intact TLR4 signaling network is essential for saturated fat–induced insulin resistance and for the induction of ceramide synthesis (89, 175). TLR4 agonists induce transcription of several enzymes in the de novo ceramide biosynthesis pathway (89, 173, 177).
TNF-α, another cytokine that is elevated in obesity, also increases ceramide levels. Studies in cultured cell reveal that it accomplishes this via coordinated activation and enhanced expression of the ceramide generating (e.g., SPT) and metabolizing enzymes (e.g., sphingomyelinase) (30, 123, 178). In cultured myotubes, we determined that TNF-α effects on ceramides are biphasic, with an acute increase resulting from activation of sphingomyelinase followed by a sustained upregulation resulting from enhanced de novo biosynthesis (S.A. Summers, unpublished observation).
Some data suggest that ceramides may themselves stimulate inflammation, which would enable a positive feedback mechanism that would exacerbate tissue damage. For example, they may activate the Nod-like receptor (Nlrp3) inflammasome, which induces caspase 1 cleavage in macrophages and adipose tissue and enhances cytokine release (179).
Endocannabinoids
Cannabinoids, the active components of marijuana (Cannabis sativa), exert their psychoactive and peripheral effects by activating Gi/o protein–coupled receptors (cannabinoid receptor CB1R) that modulate ion channels, adenylyl cyclase, and extracellular signal–regulated kinases (180). Scattered reports indicate that cannabinoid receptors may induce ceramides by enhancing sphingomyelin hydrolysis and by stimulating de novo biosynthesis (180, 181). While the actions of ceramides in neurotransmitter release are not clearly elucidated, one study has implicated this cannabinoid action in obesity-driven disease (182). In obese mice, inhibition of CB1R using the inverse agonist JD5037 or genetic deletion of hepatic CB1R reversed steatosis and improved glucose tolerance and insulin sensitivity (183). These interventions lowered ceramide levels in the liver by reducing SPT activity, decreasing transcripts encoding biosynthetic genes (i.e., Sptlc3, CerS1, and CerS6), and increasing transcripts encoding ceramidases (i.e., Asah1 and Asah2) (183). Moreover, the SPT inhibitor myriocin abrogated endocannabinoid-induced hepatic insulin resistance (183).
β-Adrenergic Agonists
In adipocytes, β-adrenergic receptors stimulate heat production by increasing mitochondrial activity and uncoupling (184). As noted above, ceramides are potent inhibitors of this thermogenic response, owing in part to their ability to inhibit mitochondrial activity and block glucose utilization (88, 185). Using a newly developed flux assay to monitor rates of ceramide production, we found that β-adrenergic agonists rapidly and completely shut down ceramide biosynthesis (12), even under conditions where exogenous fatty acids are plentiful. This work reveals a novel regulatory mechanism that controls rates of ceramide biosynthesis to control metabolic activity of adipose depots.
Adiponectin
Adiponectin, a protein hormone secreted from metabolically healthy adipocytes, elicits a broad spectrum of anti-inflammatory, antiatherogenic, and antidiabetic actions (50). For example, the adipokine improves glucose homeostasis by enhancing insulin sensitivity in adipose tissue and the liver and promoting survival of insulin-secreting beta-cells. It improves lipid handling by modulating rates of triglyceride synthesis, oxidation, and lipolysis in adipocytes and the liver. It also reduces inflammation through direct actions on immune cells. These beneficial actions were initially thought to be mediated by AMPK, a serine/threonine kinase activated during starvation (186). However, although AMPK can be activated by adiponectin, it is dispensable for most of the aforementioned physiological responses (49).
Ceramidases bear sequence homology to the receptors within the progestin and adipoQ (PAQR) superfamily, including the adiponectin receptors ADIPOR1 and ADIPOR2. Villa and colleagues (187) determined that these PAQRs promoted accumulation of the free sphingoid base in yeast. Moreover, receptor signaling can be abrogated by the inclusion of ceramidase inhibitors (188). Holland et al. (49) thus hypothesized that adiponectin might elicit its broad spectrum of metabolic improvements by degrading ceramides. These researchers demonstrated that adiponectin, via ADIPOR1 and ADIPOR2, deacylated ceramide and induced sphingosine and S1P (49, 189). They also found that mutation of critical residues in the receptor’s ceramidase motif abrogated adiponectin action (49). Proof of the bioactivity of this domain came from studies on the crystal structure of the ADIPOR isoforms. In an initial structural assessment, Tanabe et al. (190) found that human adiponectin receptors possess a hydrophobic binding pocket that resembled a comparable structure in other known ceramidases. Subsequently, Vasiliauskaite-Brooks et al. (51) elucidated the crystal structure of the receptor in the presence of ceramide, determining that it had a bound fatty acid and that the purified protein contained intrinsic ceramidase activity. These data unveiled a new paradigm in receptor signaling, revealing that PAQR ligands could alter tissue function by acutely degrading ceramides.
To test this explicitly, Scherer and colleagues (100) generated mice overexpressing AdipoR selectively in liver. The increase in hepatic AdipoR reduced hepatic ceramide content and increased AdipoR-induced ceramidase activity, leading to improved hepatic insulin resistance and resolved hepatic steatosis.
Intestinal Farnesoid X Receptors
The farnesoid X receptor (FXR) is a nuclear hormone receptor present in the liver and intestines. Bile acids, which are its primary ligands, cause FXR to translocate to the nucleus, where it suppresses expression of genes (e.g., cholesterol 7 alpha-hydroxylase) required to synthesize bile acids from cholesterol. This negative feedback pathway limits production of new bile acids when enterohepatic levels are high.
The receptor also regulates genes involved in lipid and glucose metabolism and displays a complex and multifaceted role in the development of cardiometabolic pathologies. FXR agonists are in clinical development as a means of reducing hepatic steatosis (191). However, Gonzalez and colleagues found that intestinal FXR enhanced metabolic disorders, owing to its ability to act as a sensor of the gut lumen environment. They found that intestinal FXR enhances hepatic steatosis and gluconeogenesis by stimulating intestinal ceramide biosynthesis (138, 185, 192). Specifically, FXR stimulated transcription of genes relevant to de novo sphingolipid biosynthesis, which increases circulating levels of ceramides and produces a commensurate ceramide-driven induction of genes driving fat accumulation and gluconeogenesis in the liver. These actions limit the therapeutic potential of the FXR agonists and suggest that a combination of tissue-specific pharmaceuticals may be needed.
A UNIFYING HYPOTHESIS ABOUT THE EVOLUTION OF CERAMIDES AS NUTRITIONAL GAUGES
Since fatty acids are detergent-like molecules that can perturb membrane structures, cells go to great lengths to keep free fatty acid levels low. They accomplish this by storing excess fatty acids in macromolecules, with glycerolipids, including the triglycerides, serving as the major storage forms. When the energetic needs of the tissue have been met and the storage capacity starts to fill, ceramides initiate cellular responses that change fuel preference and induce lipid storage. As described herein, they inhibit glucose and amino acid utilization and induce the uptake of fatty acids and their conversion into triglycerides. They also alter mitochondrial efficiency, which may allow cells to metabolize more fatty acids by limiting their impact on mitochondrial membrane potential. When ceramides rise to critical levels, they induce apoptosis to control the death of the compromised and unstable cell. We hypothesize that these responses are part of an evolutionary conserved adaptation to excessive fatty acids.
Though we acknowledge this as speculation, the following ceramide actions support the provocative idea that cells produce ceramides to protect themselves from the deluge of excess fat:
Ceramides limit the damage free fatty acids can do to cellular membranes by enhancing their safe passage through bilayers and enabling their esterification (e.g., via CD36) (12, 100).
Ceramides facilitate the safe storage of fatty acids by inducing expression of Srebf1, a master regulator of genes required for triglyceride synthesis and lipid droplet assembly (12, 138).
Ceramides slow release of fatty acids from lipid droplets (via HSL) (12).
Ceramides alter fuel preference by limiting the entry of glucose and amino acids into cells (7, 193-195).
Ceramides reduce mitochondrial efficiency (12, 25, 26, 98, 122, 196), which allows cells to minimize the effect of the excessive fatty acids on mitochondrial membrane potential.
Ceramides mediate fatty acid–induced apoptosis (123).
The CERS enzymes themselves, beyond producing ceramides, respond to fatty acids by repressing transcription of lipases (43, 197).
These data clearly identify ceramides as major regulators of cellular metabolism during times of fuel surplus.
OUTLOOK AND FUTURE DIRECTIONS
The astounding number of studies in numerous complementary and redundant rodent models make one thing clear: Ceramides are important nutrient signals that contribute to cardiometabolic disease. Clinical profiling studies further indicate that they are relevant to human disease. These transformative discoveries raise the exciting possibility that ceramide-lowering strategies could have therapeutic value in a wide range of cardiometabolic disease processes. Nonetheless, several important topics remain underexplored.
The precise, tissue-specific roles of ceramides are not fully defined. The availability of new tools to enable tissue-specific control of ceramide synthesis, including gain-of-function models, will likely reveal which tissues are most sensitive to ceramides.
Although several key mechanisms have been identified (e.g., ceramide regulation of AKT), we do not yet have a very satisfying explanation for how ceramides are detected in cells. For example, the reasons that the double bond in the sphingoid base and the length of the acyl chain are such critical determinants of ceramide action are largely unknown.
Little information exists about the genetic determinants of ceramide accumulation in the population. Several single nucleotide polymorphisms have been identified in ceramide-modifying enzymes. For example, CERS2 polymorphisms are associated with increased HbA1c and risk of diabetic kidney disease (198-200). This common natural variant results in the replacement of a glutamate to an alanine in an important protein domain and is predicted to be inactivating. The impact of this and other rare and common variants on sphingolipid accumulation and disease risk merits additional attention.
The regulatory elements that influence rates of ceramide production, including the activity and expression of ceramide-synthesizing and -metabolizing enzymes, are still not fully resolved.
Therapeutics to safely lower ceramides in humans have not been developed. Alternatively, insufficient information exists regarding specific behavioral interventions that lower ceramides and improve patient health.
Despite these areas of uncertainty, ceramides undoubtedly serve as a nexus in nutrient signaling networks and have profound effects on a wide variety of metabolic disease processes. Research on these exciting lipids holds great promise as a means of combating the metabolic disease epidemic.
ACKNOWLEDGMENTS
The authors receive research support from the US National Institutes of Health (NIH; DK115824, DK116450 to S.A.S.; DK115824 and DK124326 to B.C.), the Juvenile Diabetes Research Foundation (JDRF 3-SRA-2019-768-A-B to S.A.S.), the American Diabetes Association (to S.A.S.), the American Heart Association (to S.A.S.), the Margolis Foundation (to S.A.S.), the American Heart Association Career Development Award (to B.C.), and the US Department of Agriculture (2019-67018-29250 to B.C.). B.C. also received a pilot grant from the Diabetes Research Center of the NIH at Washington University in St. Louis under award number P30DK020579.
Footnotes
DISCLOSURE STATEMENT
S.A.S. is a cofounder of and consultant for Centaurus Therapeutics. B.C. is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.International Diabetes Federation. 2020. IDF Diabetes Atlas. International Diabetes Federation, Brussels: https://www.diabetesatlas.org/en/ [Google Scholar]
- 2.Spengler EK, Loomba R. 2015. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin. Proc 90:1233–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organ. 2019. Cardovascular diseases (CVDs). Fact Sheet, World Health Organ, Geneva: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- 4.Randle PJ, Garland PB, Hales CN, Newsholme EA. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–89 [DOI] [PubMed] [Google Scholar]
- 5.Shulman GI. 2000. Cellular mechanisms of insulin resistance. J. Clin. Investig 106:171–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turinsky J, O’Sullivan DM, Bayly BP. 1990. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J. Biol. Chem 265:16880–85 [PubMed] [Google Scholar]
- 7.Summers SA, Garza LA, Zhou H, Birnbaum MJ. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol 18:5457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. 2005. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem 280:20148–53 [DOI] [PubMed] [Google Scholar]
- 9.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, et al. 2003. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem 278:10297–303 [DOI] [PubMed] [Google Scholar]
- 10.Chavez JA Summers SA. 2003. Characterizing the effects of saturated fattyacids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys 419:101–9 [DOI] [PubMed] [Google Scholar]
- 11.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5:167–79 [DOI] [PubMed] [Google Scholar]
- 12.Chaurasia B, Tippetts TS, Monibas RM, Liu J, Li Y, et al. 2019. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365:386–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen TC, Lee RA, Tsai SL, Kanamaluru D, Gray NE, et al. 2019. An ANGPTL4-ceramide-protein kinase Cζ axis mediates chronic glucocorticoid exposure-induced hepatic steatosis and hypertriglyceridemia in mice. J. Biol. Chem 294:9213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correnti JM, Juskeviciute E, Swarup A, Hoek JB. 2014. Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol 306:G959–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, Garner B. 2008. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res 49:324–31 [DOI] [PubMed] [Google Scholar]
- 16.Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, et al. 2007. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol 73:1340–46 [DOI] [PubMed] [Google Scholar]
- 17.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, et al. 2005. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem 280:10284–89 [DOI] [PubMed] [Google Scholar]
- 18.Ji R, Akashi H, Drosatos K, Liao X, Jiang H, et al. 2017. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2:e82922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, et al. 2015. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLOS ONE 10:e0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurek K, Piotrowska DM, Wiesiolek-Kurek P, Łukaszuk B, Chabowski A, et al. 2014. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 34:1074–83 [DOI] [PubMed] [Google Scholar]
- 21.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res 49:2101–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, et al. 2004. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110:3465–71 [DOI] [PubMed] [Google Scholar]
- 23.Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, et al. 2006. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189:264–72 [DOI] [PubMed] [Google Scholar]
- 24.Park TS, Rosebury W, Kindt EK, Kowala MC, Panek RL. 2008. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res 58:45–51 [DOI] [PubMed] [Google Scholar]
- 25.Raichur S, Wang ST, Chan PW, Li Y, Ching J, et al. 2014. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20:687–95 [DOI] [PubMed] [Google Scholar]
- 26.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, et al. 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. CellMetab. 20:678–86 [DOI] [PubMed] [Google Scholar]
- 27.Summers SA. 2018. Could ceramides become the new cholesterol? Cell Metab. 27:276–80 [DOI] [PubMed] [Google Scholar]
- 28.Hannun YA, Obeid LM. 2018. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol 19:175–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill AH Jr., Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, et al. 1997. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol 142:208–25 [DOI] [PubMed] [Google Scholar]
- 30.Hannun YA, Obeid LM. 1995. Ceramide: an intracellular signal for apoptosis. Trends Biochem. Sci 20:73–77 [DOI] [PubMed] [Google Scholar]
- 31.Summers SA, Chaurasia B, Holland WL. 2019. Metabolic messengers: ceramides. Nat.Metab 1:1051–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill AH Jr. 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem 277:25843–46 [DOI] [PubMed] [Google Scholar]
- 33.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, et al. 2009. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. PNAS 106:8186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis D, Kannan M, Wattenberg B. 2018. Orm/ORMDLproteins: gate guardians and master regulators. Adv. Biol. Regul 70:3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lone MA, Santos T, Alecu I, Silva LC, Hornemann T. 2019. 1-Deoxysphingolipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864:512–21 [DOI] [PubMed] [Google Scholar]
- 36.Bertea M, Rutti MF, Othman A, Marti-Jaun J, Hersberger M, et al. 2010. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brozinick JT, Hawkins E, Hoang Bui H, Kuo MS, Tan B, et al. 2013. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes 37:1064–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gai Z, Gui T, Alecu I, Lone MA, Hornemann T, et al. 2019. Farnesoid X receptor activation induces the degradation of hepatotoxic 1-deoxysphingolipids in non-alcoholic fatty liver disease. Liver Int. 40:844–59 [DOI] [PubMed] [Google Scholar]
- 39.Othman A, Bianchi R, Alecu I, Wei Y, Porretta-Serapiglia C, et al. 2015. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes 64:1035–45 [DOI] [PubMed] [Google Scholar]
- 40.Othman A, Rutti MF, Ernst D, Saely CH, Rein P, et al. 2012. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia 55:421–31 [DOI] [PubMed] [Google Scholar]
- 41.Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, et al. 2015. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 3:e000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelnik ID, Rozman B, Rosenfeld-Gur E, Ben-Dor S, Futerman AH. 2019. A stroll down the CerS lane. Adv. Exp. Med. Biol 1159:49–63 [DOI] [PubMed] [Google Scholar]
- 43.Sociale M, Wulf AL, Breiden B, Klee K, Thielisch M, et al. 2018. Ceramide synthase schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep. 22:967–78 [DOI] [PubMed] [Google Scholar]
- 44.Siddique MM, Li Y, Chaurasia B, Kaddai VA, Summers SA. 2015. Dihydroceramides: from bit players to lead actors. J. Biol. Chem 290:15371–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizutani Y, Kihara A, Igarashi Y. 2004. Identification of the human sphingolipid C4-hydroxylase, hDES2, and its up-regulation during keratinocyte differentiation. FEBS Lett. 563:93–97 [DOI] [PubMed] [Google Scholar]
- 46.Omae F, Miyazaki M, Enomoto A, Suzuki M, Suzuki Y, Suzuki A. 2004. DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem. J 379:687–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, et al. 2017. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 25:686–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coant N, Sakamoto W, Mao C, Hannun YA. 2017. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul 63:122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, et al. 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med 17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straub LG, Scherer PE. 2019. Metabolic messengers: adiponectin. Nat. Metab 1:334–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasiliauskaite-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, et al. 2017. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544:120–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cartier A, Hla T. 2019. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366:eaar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulgaropoulos B, Amenitsch H, Laggner P, Pabst G. 2010. Implication of sphingomyelin/ceramide molar ratio on the biological activity of sphingomyelinase. Biophys. J 99:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claus RA, Dorer MJ, Bunck AC, Deigner HP. 2009. Inhibition of sphingomyelin hydrolysis: targeting the lipid mediator ceramide as a key regulator of cellular fate. Curr. Med. Chem 16:1978–2000 [DOI] [PubMed] [Google Scholar]
- 55.Kitatani K, Idkowiak-Baldys J, Hannun YA. 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 20:1010–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, et al. 2009. Involvement of acid β-glucosidase 1 in the salvage pathway of ceramide formation. J. Biol. Chem 284:12972–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, et al. 2019. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: The Strong Heart Family Study. EBioMedicine 41:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, et al. 2018. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 67:1663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorens B, Rodriguez A, Cruciani-Guglielmacci C, Wigger L, Ibberson M, Magnan C. 2019. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—a step towards precision medicine. Mol. Metab 27S:S147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wigger L, Cruciani-Guglielmacci C, Nicolas A, Denom J, Fernandez N, et al. 2017. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 18:2269–79 [DOI] [PubMed] [Google Scholar]
- 61.de Carvalho LP, Tan SH, Ow GS, Tang Z, Ching J, et al. 2018. Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Transl. Sci 3:163–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, et al. 2019. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J 41:371–80 [DOI] [PubMed] [Google Scholar]
- 63.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, et al. 2016. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J 37:1967–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, et al. 2018. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc 7 10.1161/JAHA.117.007931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, et al. 2016. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol 36:2424–30 [DOI] [PubMed] [Google Scholar]
- 66.Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, et al. 2020. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig 130:1363–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huynh K, Barlow CK, Jayawardana KS, Weir JM, Mellett NA, et al. 2019. High-throughput plasma lipidomics: detailed mapping of the associations with cardiometabolic risk factors. Cell Chem. Biol 26:71–84.e4 [DOI] [PubMed] [Google Scholar]
- 68.Luukkonen PK, Sadevirta S, Zhou Y, Kayser B, Ali A, et al. 2018. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 41:1732–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luukkonen PK, Zhou Y, Sadevirta S, Leivonen M, Arola J, et al. 2016. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol 64:1167–75 [DOI] [PubMed] [Google Scholar]
- 70.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, et al. 2007. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56:1960–68 [DOI] [PubMed] [Google Scholar]
- 71.Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, et al. 2010. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coen PM, Goodpaster BH. 2012. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab 23:391–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, et al. 2013. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity 21:2362–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dube JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, et al. 2011. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54:1147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, et al. 2004. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- 76.Mantovani A, Bonapace S, Lunardi G, Canali G, Dugo C, et al. 2020. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabetes Metab. 46:150–57 [DOI] [PubMed] [Google Scholar]
- 77.Karjalainen JP, Mononen N, Hutri-Kahonen N, Lehtimaki M, Hilvo M, et al. 2019. New evidence from plasma ceramides links apoE polymorphism to greater risk of coronary artery disease in Finnish adults. J. Lipid Res 60:1622–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, et al. 2020. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J 41:371–80 [DOI] [PubMed] [Google Scholar]
- 79.Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, et al. 2018. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res 59:1729–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng JM, Suoniemi M, Kardys I, Vihervaara T, de Boer SP, et al. 2015. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243:560–66 [DOI] [PubMed] [Google Scholar]
- 81.Hilvo M, Simolin H, Metso J, Ruuth M, Oorni K, et al. 2018. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 269:159–65 [DOI] [PubMed] [Google Scholar]
- 82.Mantovani A, Bonapace S, Lunardi G, Salgarello M, Dugo C, et al. 2018. Association of plasma ceramides with myocardial perfusion in patients with coronary artery disease undergoing stress myocardial perfusion scintigraphy. Arterioscler. Thromb. Vasc. Biol 38:2854–61 [DOI] [PubMed] [Google Scholar]
- 83.Pan W, Yu J, Shi R, Yan L, Yang T, et al. 2014. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis 25:230–35 [DOI] [PubMed] [Google Scholar]
- 84.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, et al. 2014. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab 99:E45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ng TW, Ooi EM, Watts GF, Chan DC, Meikle PJ, Barrett PH. 2015. Association of plasma ceramides and sphingomyelin with VLDL apoB-100 fractional catabolic rate before and after rosuvastatin treatment. J. Clin. Endocrinol. Metab 100:2497–501 [DOI] [PubMed] [Google Scholar]
- 86.Mayo Clinic Laboratories. 2016. Ceramides, plasma [a test in focus]. Mayo Clinic Laboratories, July 28 https://news.mayocliniclabs.com/2016/07/28/ceramides-plasma-a-test-in-focus/ [Google Scholar]
- 87.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, et al. 2012. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125:2844–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, et al. 2016. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24:820–34 [DOI] [PubMed] [Google Scholar]
- 89.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig 121:1858–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, et al. 2012. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61:1848–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, et al. 2010. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59:2453–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang M, Li C, Liu Q, Wang A, Lei M. 2019. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front. Endocrinol 10:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dekker MJ, Baker C, Naples M, Samsoondar J, Zhang R, et al. 2013. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis 228:98–109 [DOI] [PubMed] [Google Scholar]
- 94.Shimabukuro M, Zhou YT, Levi M, Unger RH. 1998. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. PNAS 95:2498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, et al. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–62 [DOI] [PubMed] [Google Scholar]
- 96.Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, et al. 2012. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem 287:17426–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. 2009. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab 297:E1420–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammerschmidt P, Ostkotte D, Nolte H, Gerl MJ, Jais A, et al. 2019. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177:1536–52.e23 [DOI] [PubMed] [Google Scholar]
- 99.Turpin-Nolan SM, Hammerschmidt P, Chen W, Jais A, Timper K, et al. 2019. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26:1–10.e7 [DOI] [PubMed] [Google Scholar]
- 100.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, et al. 2015. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. CellMetab. 22:266–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chakraborty M, Lou C, Huan C, Kuo MS, Park TS, et al. 2013. Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis. J. Clin. Investig 123:1784–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, et al. 2015. Ceramide-initiated protein phosphatase 2a activation contributes to arterial dysfunction in vivo. Diabetes 64:3914–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reforgiato MR, Milano G, Fabrias G, Casas J, Gasco P, et al. 2016. Inhibition of ceramide de novo synthesis as a postischemic strategy to reduce myocardial reperfusion injury. Basic Res. Cardiol 111:12. [DOI] [PubMed] [Google Scholar]
- 104.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, et al. 2012. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Investig 122:3919–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raichur S, Brunner B, Bielohuby M, Hansen G, Pfenninger A, et al. 2019. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol. Metab 21:36–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gosejacob D, Jäger PS, Vom Dorp K, Frejno M, Carstensen AC, et al. 2016. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem 291:6989–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner N, Lim XY, Toop HD, Osborne B, Brandon AE, et al. 2018. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun 9:3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Z, Zhang H, Liu J, Liang CP, Li Y, et al. 2011. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol 31:4205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park M, Kaddai V, Ching J, Fridianto KT, Sieli RJ, et al. 2016. A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes. J. Biol. Chem 291:23978–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bijl N, Sokolovic M, Vrins C, Langeveld M, Moerland PD, et al. 2009. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 50:1431–41 [DOI] [PubMed] [Google Scholar]
- 111.Zhao H, Przybylska M, Wu IH, Zhang J, Maniatis P, et al. 2009. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology 50:85–93 [DOI] [PubMed] [Google Scholar]
- 112.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, et al. 2007. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56:1210–18 [DOI] [PubMed] [Google Scholar]
- 113.Chatterjee S, Bedja D, Mishra S, Amuzie C, Avolio A, et al. 2014. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/− mice and rabbits fed a high-fat and -cholesterol diet. Circulation 129:2403–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bietrix F, Lombardo E, van Roomen CP, Ottenhoff R, Vos M, et al. 2010. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol 30:931–37 [DOI] [PubMed] [Google Scholar]
- 115.Glaros EN, Kim WS, Rye KA, Shayman JA, Garner B. 2008. Reduction of plasma glycosphingolipid levels has no impact on atherosclerosis in apolipoprotein E-null mice. J. Lipid Res 49:1677–81 [DOI] [PubMed] [Google Scholar]
- 116.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, et al. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. PNAS 104:13678–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inokuchi J 2007. Insulin resistance as a membrane microdomain disorder. Yakugaku Zasshi 127:579–86 [DOI] [PubMed] [Google Scholar]
- 118.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, et al. 2003. Enhanced insulin sensitivity in mice lacking ganglioside GM3. PNAS 100:3445–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. 2014. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem 289:723–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gudz TI, Tserng KY, Hoppel CL. 1997. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem 272:24154–48 [DOI] [PubMed] [Google Scholar]
- 121.Di Paola M, Cocco T, Lorusso M. 2000. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39:6660–68 [DOI] [PubMed] [Google Scholar]
- 122.Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, et al. 2013. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem 288:4947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Obeid LM, Linardic CM, Karolak LA, Hannun YA. 1993. Programmed cell death induced by ceramide. Science 259:1769–71 [DOI] [PubMed] [Google Scholar]
- 124.Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. 2010. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis 15:553–62 [DOI] [PubMed] [Google Scholar]
- 125.Dadsena S, Bockelmann S, Mina JGM, Hassan DG, Korneev S, et al. 2019. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun 10:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Siskind LJ, Kolesnick RN, Colombini M. 2006. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6:118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. 2003. Enlargement and contracture of C2-ceramide channels. Biophys. J 85:1560–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siskind LJ, Kolesnick RN, Colombini M. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem 277:26796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siskind LJ, Colombini M. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem 275:38640–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. 2000. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci 15:156–69 [DOI] [PubMed] [Google Scholar]
- 131.Teruel T, Hernandez R, Lorenzo M. 2001. Ceramide mediates insulin resistance by tumor necrosis factor-α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50:2563–71 [DOI] [PubMed] [Google Scholar]
- 132.Zinda MJ, Vlahos CJ, Lai MT. 2001. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem. Biophys. Res. Commun 280:1107–15 [DOI] [PubMed] [Google Scholar]
- 133.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. 1993. Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem 268:15523–30 [PubMed] [Google Scholar]
- 134.Powell DJ, Hajduch E, Kular G, Hundal HS. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ -dependent mechanism. Mol. Cell. Biol 23:7794–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. 2004. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem. J 382:619–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, et al. 2010. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59:600–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stratford S, Hoehn KL, Liu F, Summers SA. 2004. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem 279:36608–15 [DOI] [PubMed] [Google Scholar]
- 138.Jiang C, Xie C, Li F, Zhang L, Nichols RG, et al. 2015. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig 125:386–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alexaki A, Clarke BA, Gavrilova O, Ma Y, Zhu H, et al. 2017. De novo sphingolipid biosynthesis is required for adipocyte survival and metabolic homeostasis. J. Biol. Chem 292:3929–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee SY, Lee HY, Song JH, Kim GT, Jeon S, et al. 2017. Adipocyte-specific deficiency of de novo sphingolipid biosynthesis leads to lipodystrophy and insulin resistance. Diabetes 66:2596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, et al. 2011. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13:249–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. 2010. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151:2933–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brown MS, Goldstein JL. 2008. Selective versus total insulin resistance: a pathogenic paradox. CellMetab. 7:95–96 [DOI] [PubMed] [Google Scholar]
- 144.Petersen MC, Jurczak MJ. 2016. CrossTalk opposing view: intramyocellular ceramide accumulation does not modulate insulin resistance. J. Physiol 594:3171–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Summers SA, Goodpaster BH. 2016. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol 594:3167–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu W, Ross J, Geng T, Brice SE, Cowart LA. 2011. Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. J. Biol. Chem 286:16596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Watson ML, Coghlan M, Hundal HS. 2009. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem. J 417:791–801 [DOI] [PubMed] [Google Scholar]
- 148.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, et al. 2013. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62:401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. 1998. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem 273:32487–90 [DOI] [PubMed] [Google Scholar]
- 150.Boslem E, Meikle PJ, Biden TJ. 2012. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets 4:177–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. 2003. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J. Biol. Chem 278:30015–21 [DOI] [PubMed] [Google Scholar]
- 152.Sjöholm A 1995. Ceramide inhibits pancreatic β-cell insulin production and mitogenesis and mimics the actions of interleukin-1β. FEBS Lett. 367:283–86 [DOI] [PubMed] [Google Scholar]
- 153.Veret J, Bellini L, Giussani P, Ng C, Magnan C, Le Stunff H. 2014. Roles of sphingolipid metabolism in pancreatic β cell dysfunction induced by lipotoxicity. J. Clin. Med 3:646–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu Q, Shan X, Miao H, Lu Y, Xu J, et al. 2009. Acute activation of acid ceramidase affects cytokine-induced cytotoxicity in rat islet β-cells. FEBS Lett. 583:2136–41 [DOI] [PubMed] [Google Scholar]
- 155.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, et al. 2003. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 144:4154–63 [DOI] [PubMed] [Google Scholar]
- 156.Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. 2018. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front. Endocrinol 9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tian G, Sol ER, Xu Y, Shuai H, Tengholm A. 2015. Impaired cAMP generation contributes to defective glucose-stimulated insulin secretion after long-term exposure to palmitate. Diabetes 64:904–15 [DOI] [PubMed] [Google Scholar]
- 158.Lucchetta M, Rudilosso S, Costa S, Bruttomesso D, Ruggero S, et al. 2010. Anti-ganglioside autoantibodies in type 1 diabetes. Muscle Nerve 41:50–53 [DOI] [PubMed] [Google Scholar]
- 159.Dotta F, Falorni A, Tiberti C, Dionisi S, Anastasi E, et al. 1997. Autoantibodies to the GM2–1 islet ganglioside and to GAD-65 at type 1 diabetes onset. J. Autoimmun 10:585–88 [DOI] [PubMed] [Google Scholar]
- 160.Misasi R, Dionisi S, Farilla L, Carabba B, Lenti L, et al. 1997. Gangliosides and autoimmune diabetes. Diabetes Metab. Rev 13:163–79 [DOI] [PubMed] [Google Scholar]
- 161.Dionisi S, Dotta F, Diaz-Horta O, Carabba B, Viglietta V, Di Mario U. 1997. Target antigens in autoimmune diabetes: pancreatic gangliosides. Ann. Inst. Super. Sanita 33:433–35 [PubMed] [Google Scholar]
- 162.Park SK, La Salle DT, Cerbie J, Cho JM, Bledsoe A, et al. 2019. Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans. Am. J. Physiol. Heart Circ. Physiol 316:H106–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li W, Yang X, Xing S, Bian F, Yao W, et al. 2014. Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med. Cell. Longev 2014:823071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao D, Pararasa C, Dunston CR, Bailey CJ, Griffiths HR. 2012. Palmitate promotes monocyte atherogenicity via de novo ceramide synthesis. Free Radic. Biol. Med 53:796–806 [DOI] [PubMed] [Google Scholar]
- 165.Contreras C, Gonzalez-Garcia I, Martinez-Sanchez N, Seoane-Collazo P, Jacas J, et al. 2014. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 9:366–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campana M, Bellini L, Rouch C, Rachdi L, Coant N, et al. 2018. Inhibition of central de novo ceramide synthesis restores insulin signaling in hypothalamus and enhances β-cell function of obese Zucker rats. Mol. Metab 8:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nordstrom V, Willershauser M, Herzer S, Rozman J, von Bohlenund Halbach O, et al. 2013. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLOS Biol. 11:e1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Herzer S, Meldner S, Grone HJ, Nordström V. 2015. Fasting-induced lipolysis and hypothalamic insulin signaling are regulated by neuronal glucosylceramide synthase. Diabetes 64:3363–76 [DOI] [PubMed] [Google Scholar]
- 169.Chaurasia B, Summers SA. 2015. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab 26:538–50 [DOI] [PubMed] [Google Scholar]
- 170.Stockli J, Fisher-Wellman KH, Chaudhuri R, Zeng XY, Fazakerley DJ, et al. 2017. Metabolomic analysis of insulin resistance across different mouse strains and diets. J. Biol. Chem 292:19135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–67 [DOI] [PubMed] [Google Scholar]
- 172.de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, et al. 2009. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52:2612–15 [DOI] [PubMed] [Google Scholar]
- 173.Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, et al. 2013. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem 288:2923–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Majumdar I, Mastrandrea LD. 2012. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 41:442–49 [DOI] [PubMed] [Google Scholar]
- 175.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig 116:3015–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, et al. 2018. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 27:1096–110.e5 [DOI] [PubMed] [Google Scholar]
- 177.Sims K, Haynes CA, Kelly S, Allegood JC, Wang E, et al. 2010. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem 285:38568–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kolesnick R 1992. Ceramide: a novel second messenger. Trends Cell Biol. 2:232–36 [DOI] [PubMed] [Google Scholar]
- 179.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, et al. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med 17:179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guzman M, Galve-Roperh I, Sanchez C. 2001. Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol. Sci 22:19–22 [DOI] [PubMed] [Google Scholar]
- 181.Velasco G, Galve-Roperh I, Sanchez C, Blazquez C, Haro A, Guzman M. 2005. Cannabinoids and ceramide: two lipids acting hand-by-hand. Life Sci. 77:1723–31 [DOI] [PubMed] [Google Scholar]
- 182.Gatta-Cherifi B, Cota D. 2016. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int. J. Obes 40:210–19 [DOI] [PubMed] [Google Scholar]
- 183.Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, et al. 2014. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59:143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ikeda K, Maretich P, Kajimura S. 2018. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab 29:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang C, Xie C, Lv Y, Li J, Krausz KW, et al. 2015. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun 6:10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, et al. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med 8:1288–95 [DOI] [PubMed] [Google Scholar]
- 187.Villa NY, Kupchak BR, Garitaonandia I, Smith JL, Alonso E, et al. 2009. Sphingolipids function as downstream effectors of a fungal PAQR. Mol. Pharmacol 75:866–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kupchak BR, Garitaonandia I, Villa NY, Smith JL, Lyons TJ. 2009. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFα and a ceramidase inhibitor. Biochemistry 48:5504–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, et al. 2017. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab 6:267–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, et al. 2015. Crystal structures of the human adiponectin receptors. Nature 520:312–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xi Y, Li H. 2020. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother 121:109609. [DOI] [PubMed] [Google Scholar]
- 192.Xie C, Jiang C, Shi J, Gao X, Sun D, et al. 2017. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66:613–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang CN, O’Brien L, Brindley DN. 1998. Effects of cell-permeable ceramides and tumor necrosis factor-α on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes 47:24–31 [DOI] [PubMed] [Google Scholar]
- 194.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. 2005. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 19:461–63 [DOI] [PubMed] [Google Scholar]
- 195.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. 2008. Ceramide starves cells to death by downregulating nutrient transporter proteins. PNAS 105:17402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kogot-Levin A, Saada A. 2014. Ceramide and the mitochondrial respiratory chain. Biochimie 100:88–94 [DOI] [PubMed] [Google Scholar]
- 197.Chaurasia B, Holland WL, Summers SA. 2018. Does this schlank make me look fat? Trends Endocrinol. Metab 29:597–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shiffman D, Pare G, Oberbauer R, Louie JZ, Rowland CM, et al. 2014. A gene variant in CERS2 is associated with rate of increase in albuminuria in patients with diabetes from ONTARGET and TRANSCEND. PLOS ONE 9:e106631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, et al. 2010. New loci associated with kidney function and chronic kidney disease. Nat. Genet 42:376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Price PM, Hirschhorn K, Safirstein RL. 2010. Chronic kidney disease and GWAS: “the proper study of mankind is man.” Cell Metab. 11:451–52 [DOI] [PubMed] [Google Scholar]
- 201.Hojjati MR, Li Z, Jiang XC. 2005. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta 1737:44–51 [DOI] [PubMed] [Google Scholar]
- 202.Ohta E, Ohira T, Matsue K, Ikeda Y, Fujii K, et al. 2009. Analysis of development of lesions in mice with serine palmitoyltransferase (SPT) deficiency—Sptlc2 conditional knockout mice. Exp. Anim 58:515–24 [DOI] [PubMed] [Google Scholar]
- 203.Li Z, Kabir I, Tietelman G, Huan C, Fan J, et al. 2018. Sphingolipid de novo biosynthesis is essential for intestine cell survival and barrier function. Cell Death Dis. 9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, et al. 2012. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet 21:586–608 [DOI] [PubMed] [Google Scholar]
- 205.Ebel P, Imgrund S, Vom Dorp K, Hofmann K, Maier H, et al. 2014. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. Biochem. J 461:147–58 [DOI] [PubMed] [Google Scholar]
- 206.Jennemann R, Kaden S, Sandhoff R, Nordstrom V, Wang S, et al. 2012. Glycosphingolipids are essential for intestinal endocytic function. J. Biol. Chem 287:32598–616 [DOI] [PMC free article] [PubMed] [Google Scholar]