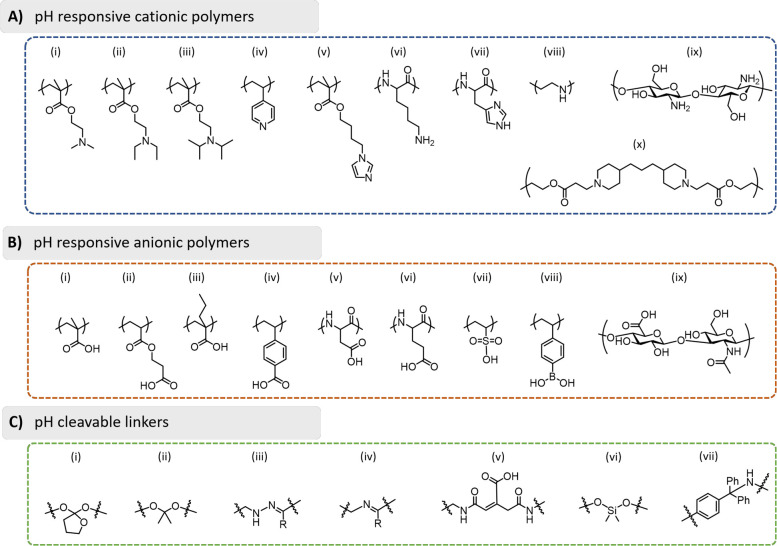
Figure 8.
Summary of the functional groups and linkers used in the development of pH-sensitive materials. (A) pH-responsive cationic polymers: (i) poly(2-dimethylaminoethyl methacrylate) (PDMAEMA), (ii) poly(2-diethylaminoethyl methacrylate) (PDEAEMA), (iii) poly(2-diisopropylaminoethyl methacrylate) (PDPAEMA), (iv) poly(4-vinylpyridine) (PVP), (v) poly(4-(1H-imidazol-1-yl)butyl methacrylate (PImBuMA), (vi) poly(lysine) (PLys), (vii) poly(histidine) (PHis), (viii) poly(ethylenimine) (PEI), (ix) chitosan, and (x) poly(β-amino ester) (PbAE). (B) pH-responsive anionic polymers: (i) poly(acrylic acid) (PAA), (ii) poly(2-carboxyethyl acrylate) (PCEA), (iii) poly(2-propylacrylic acid) (PPAA), (iv) poly(4-vinylbenzoic acid) (PVBA), (v) poly(aspartic acid) (PAsA), (vi) poly(glutamic acid) (PGA), (vii) poly(vinylsulfonic acid) (PVSA), (viii) poly(vinylphenylboronic acid) (PVPBA), and (ix) hyaluronic acid. (C) pH-cleavable linkers: (i) ortho-esters, (ii) ketals/acetals, (iii) hydrazones, (iv) imines, (v) maleic acid amide derivatives (including cis-aconityl shown), (vi) silyl ethers, and (vii) trityl derivatives.