Fig. 1 |. Structures of human ferroportin.
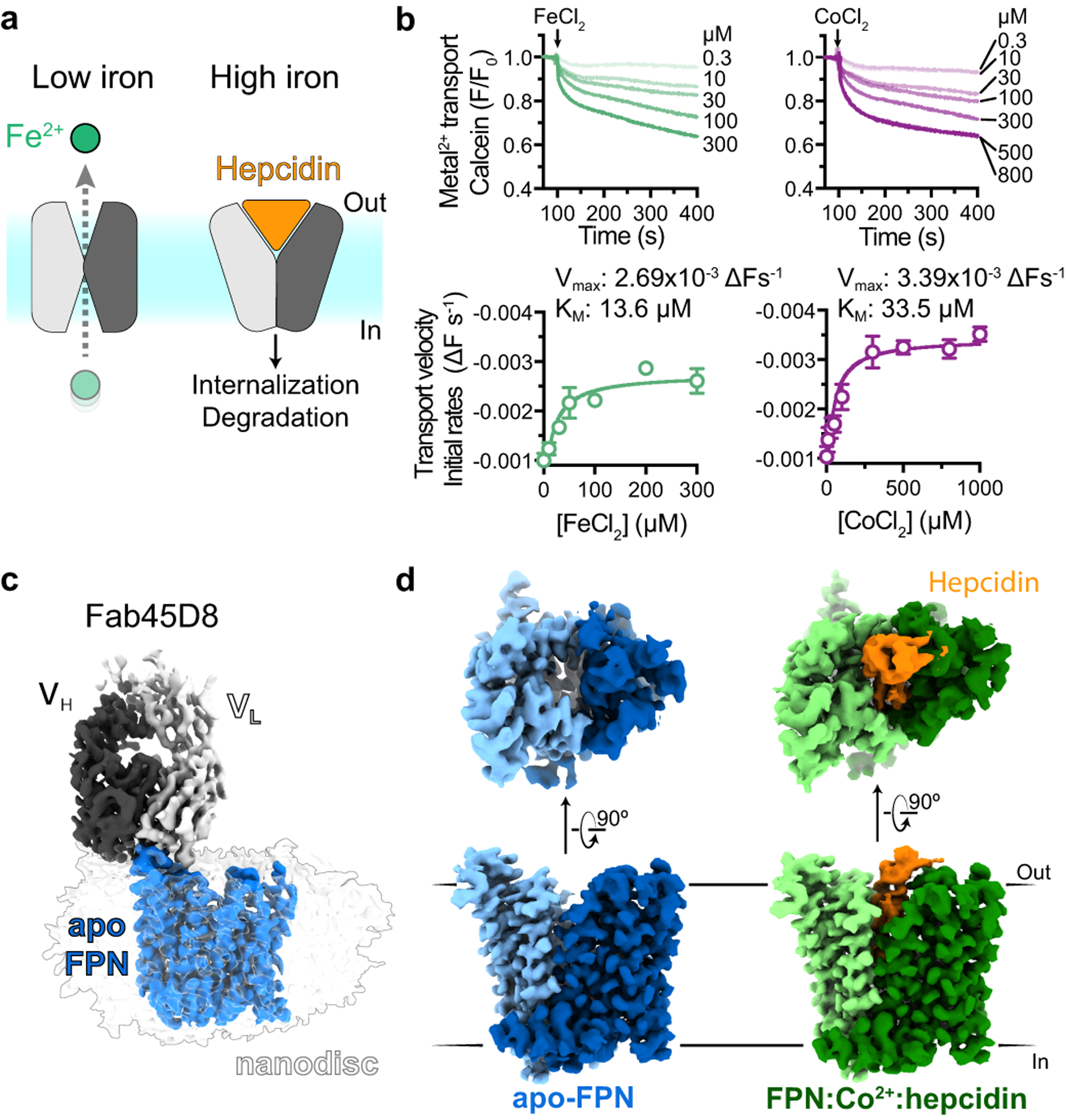
a, Ferroportin effluxes cellular iron (Fe2+) by an alternating access mechanism. Hepcidin binds to outward-open ferroportin and induces ubiquitination and degradation. b, Transport of Fe2+ and Co2+ by human ferroportin reconstituted into calcein containing liposomes. Time-course experiments were performed by addition of ions (arrow) and the steady-state kinetic analysis was performed by fitting initial rates, obtained from the linear phase of transport, to the Michaelis-Menten function. Time-course data are representative experiments, and data points are means +/− s.e.m. from n = 3 independent experiments. c, Cryo-EM map of apo-FPN-Fab45D8 complex in lipid nanodisc. d, Cryo-EM density of apo and Co2+/hepcidin bound FPN. The N and C domains are colored in different shades of blue for apo-FPN and green for Co2+/hepcidin bound FPN. Hepcidin (orange) binds to an extracellular facing cavity in FPN.
