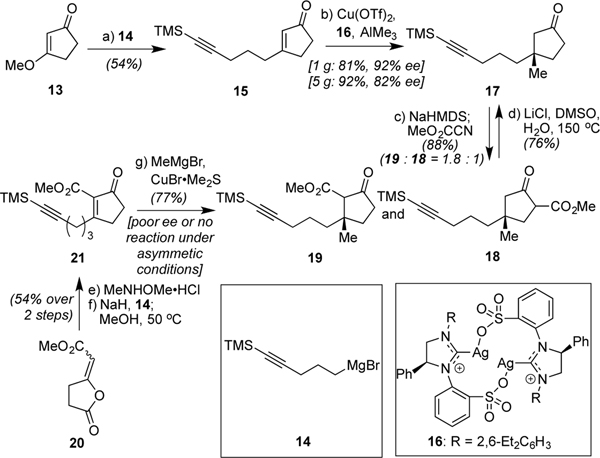
Scheme 2.
Asymmetric introduction of the guiding quaternary carbon: (a) (5-bromo-1-pentynyl)trimethylsilane (1.0 equiv), Mg (2.0 equiv), I2 (trace), THF, reflux, 4 h; 13, 0 to 23 °C, 10 h, 54%; (b) 16 (3.75 mol %), Cu(OTf)2 (7.5 mol %), THF, 23 °C, 10 min, then AlMe3 (3.0 equiv), 15, −78 °C, 12 h, 1 g scale: 81%, 92% ee; 5 g scale: 92%, 82% ee; (c) NaHMDS (2.3 equiv), THF, 0 °C, 2 h, then Mander’s reagent (1.6 equiv), −78 °C, 3 h, 32% 18; 56% 19 (d) LiCl (2 equiv), H2O (5 equiv), DMSO, 150 °C, 3 h, 76%; (e) MeNHOMe•HCl (1.3 equiv), pyridine (5.0 equiv), CH2Cl2, 23 °C, 6 h; (f) NaH (1.3 equiv), THF, 0 °C, 30 min, then 14 (1.3 equiv), −78 to 0 °C, 1 h then MeOH, 50 °C, 1 h, 54% overall; (g) CuBr•Me2S (1.2 equiv), MeMgBr (2.4 equiv), THF, −40 °C, 30 min, then 21, 2 h, 77%.