Abstract
Background
Seizures are common among patients with low-grade glioma (LGG) and can significantly affect morbidity. We sought to determine the association between the clinical and molecular factors with seizure incidence and refractoriness in LGG patients.
Methods
We conducted a retrospective review at the University of Virginia in patients with LGG (World Health Organization, WHO Grade II) evaluated between 2002 and 2015. Descriptive statistics were calculated for variables of interest, and the Kaplan-Meier method was used to estimate survival curves, which were compared with the log-rank test.
Results
A total of 291 patients were included; 254 had molecular testing performed for presence of an isocitrate dehydrogenase (IDH) mutation and/or 1p/19q codeletion. Sixty-eight percent of patients developed seizures prior to LGG diagnosis; 41% of all patients had intractable seizures. Using WHO 2016 integrated classification, there was no significant difference in seizure frequency during preoperative and postoperative periods or in developing intractable seizures, though a trend toward increased preoperative seizure incidence among patients with the IDH mutation was identified (P = .09). Male sex was significantly associated with higher seizure incidence during preoperative (P < .001) and postoperative periods (P < .001); men were also more likely to develop intractable seizures (P = .01).
Conclusions
Seizures are common among patients with LGG. Differences in preoperative or postoperative and intractable seizure rates by WHO 2016 classification were not detected. Our data showed a trend toward higher seizure incidence preoperatively in patients with IDH-mutant LGG. We describe a unique association between male sex and seizure incidence and intractability that warrants further study.
Keywords: IDH mutation, low-grade glioma, seizure, WHO 2016 classification
Tumor-related epilepsy is common among adult patients with gliomas. Seizure incidence is associated with tumor grade and is most common among patients with low-grade glioma (LGG), affecting more than 80%.1,2 Though seizures are common among patients with LGG, seizure control and frequency vary substantially. Seizures and antiepileptic drug (AED) therapy can significantly impair cognitive function and negatively affect quality of life, of particular concern given the relatively long survival of patients with LGG.3–5 Thus, adequate seizure control is an important treatment goal in this population.
Prior studies have evaluated different patient clinical and tumor molecular characteristics among LGG patients potentially contributing to seizure risk, with conflicting results on the importance of isocitrate dehydrogenase (IDH) mutations, tumor subtype (particularly following the introduction of molecular genetics in the 2016 World Health Organization [WHO] reclassification of gliomas), tumor location, extent of tumor resection, and seizure type.2,6–11 We therefore evaluated the potential impact of various clinical characteristics, histopathology, molecular characteristics (IDH mutational status and 1p/19q codeletion status), and the WHO 2016 integrated classification to preoperative and postoperative seizure risk and long-term seizure control.
Methods
Patients
We retrospectively reviewed clinical characteristics, outcome, tumor molecular characteristics, seizure incidence (preoperative and postoperative), and number of AEDs required for adequate seizure control in 291 patients with histopathologic grade II glioma based on the WHO 2007 classification evaluated at the University of Virginia between February 1, 2002 and January 1, 2015. This study was approved by the University of Virginia Institutional Review Board with a waiver of written informed consent. Patients included in this study were identified through a prospectively collected database at University of Virginia that was previously approved for research purposes through the University of Virginia Institutional Review Board. Patients age 18 years or older who met the histopathologic criteria of LGG and who underwent surgery, pathology review, and/or tumor treatment at University of Virginia were included. Patients also had to have follow-up at University of Virginia for at least 1 month postoperatively to be included. Patient baseline characteristics and tumor information potentially influencing seizure risk were assessed, including sex, age, tumor location and laterality, IDH mutation and 1p/19q codeletion status, surgery type, and histologic and molecular classification. Extent of resection (EOR) was assessed from operative notes and postoperative MRI studies.
Isocitrate Dehydrogenase and 1p19q Status
A retrospective analysis of all cases was performed in an attempt to reclassify tumors according to the WHO 2016 integrated classification. IDH mutation was determined through standard practice at the University of Virginia at the time of tumor resection, initially by immunohistochemistry (IHC) testing for IDH R132H mutant protein; in some cases of negative IHC testing for IDH R132H, polymerase chain reaction testing for noncanonical IDH1 or IDH2 mutation was performed. Identification of 1p/19q codeletion was determined by fluorescence in situ hybridization technique. One hundred patients whose tumors had an unknown IDH status at study onset were retrospectively evaluated for IDH R132H mutation by IHC testing.
Seizures
Through chart review, each patient was classified as having preoperative seizure(s) or not and/or postoperative seizure(s) or not. Preoperative seizures are seizures that occur at initial presentation until prior to surgery, whereas post-operative seizures are seizures that occur after surgery until death or last follow-up. We also identified patients with intractable seizures throughout the course of disease. Intractable seizures were identified per the International League Against Epilepsy definition as patients without adequate seizure control after trial of 2 or more AEDs either in monotherapy or combination.12 Seizure semiology was characterized as generalized-onset, focal-onset, and focal-onset with secondary generalization seizures.13
Statistical Analysis
For each event of interest (preoperative seizure status, postoperative seizure status, and intractable seizure status), frequencies and percentages were calculated by levels of other factors and chi-square tests of association were performed to test for the presence of associations. The Kaplan-Meier method was used to estimate survival by baseline factors and comparisons were performed with the log-rank test.
Results
Patient Characteristics
A total of 291 patients with LGG were included in the study (Table 1). The median age at tumor diagnosis (first surgery) was 40 years, while the median age of patients when calculated from the time of their first MRI was 39 years. The median follow-up was 69 months. LGG occurred more frequently in men than in women (54% vs 46%). The most common histologic diagnosis was oligodendroglioma (47%), followed by mixed oligoastrocytoma (25%), and astrocytoma (21%), by the WHO 2007 classification. Among 254 (87%) patients with molecular testing, 109 (43%) had IDH mutation and intact chromosomes 1p/19q (ie, diffuse astrocytoma, IDH mutant, WHO grade II, WHO 2016 classification), 92 (36%) harbored IDH mutation and 1p/19q codeletion (ie, oligodendroglioma, WHO grade II), and 53 (21%) patients had IDH wild-type LGG (ie, diffuse astrocytoma, IDH wild-type, WHO grade II). The location of LGG was categorized as temporal lobe (25% including insula), nontemporal lobe (67% including tumors in the frontal, parietal, and occipital lobes), and other locations (7%), comprising deep and midline structures, brainstem, and cerebellum. No patients with tumors in the basal ganglia, brainstem, corpus callosum, or pineal region had a seizure prior to surgery. Because these locations (“other” category) are very unlikely to cause seizures, these patients were excluded in the univariate analysis.
Table 1.
Characteristics of 291 Patients With Low-Grade Glioma
| Factors | No. (%) |
|---|---|
| Total patients | 291 |
| Median age at first surgery, y | 40 |
| Median age at first MRI, y | 39 |
| Male | 158 (54%) |
| Histologic classification, WHO 2007 | |
| Oligodendroglioma | 137 (47%) |
| Astrocytoma | 61 (21%) |
| Oligoastrocytoma | 74 (25%) |
| NOS | 19 (7%) |
| Integrated classification, WHO 2016 | |
| Oligodendroglioma, IDH-mutated, 1p/19q codeleted | 92 (36%) |
| Diffuse astrocytoma, IDH-mutated, 1p/19q intact | 109 (43%) |
| Diffuse astrocytoma, IDH wild-type | 53 (21%) |
| Not tested | 37 (NA) |
| Tumor location | |
| Temporal and insula | 74 (25%) |
| Non-temporal (frontal, parietal, and occipital lobes) | 196 (67%) |
| Other (midline and deep structures, brainstem, and cerebellum) | 21 (7%) |
| Patients with preoperative seizures | 197 (68%) |
| With postoperative seizures | 143 (73%) |
| Without postoperative seizures | 54 (27%) |
| Patients with postoperative seizures | 183 (63%) |
| With preoperative seizures | 143 (78%) |
| Without preoperative seizures | 40 (22%) |
| Patients with intractable seizures | 120 (41%) |
| Seizure types | |
| Generalized onset | 81 (41%) |
| Focal onset | 79 (40%) |
| Focal onset with secondary generalization | 23 (12%) |
| Unclear semiology | 14 (7%) |
| Types of surgery | |
| Gross total resection/lobectomy | 41 (14%) |
| Subtotal resection | 128 (44%) |
| Biopsy | 120 (42%) |
| Undetermined | 2 (NA) |
Abbreviations: IDH, isocitrate dehydrogenase; NA, not applicable; NOS, not otherwise specified; WHO, World Health Organization.
The majority of patients (68%) developed seizures before LGG diagnosis. Generalized onset was the most common seizure type (41%), followed by focal-onset seizures (40%), and focal onset with secondary generalization (12%). Forty-four percent of patients underwent subtotal resection and 42% biopsy; 14% underwent gross total resection of their tumors. Of the 68% (197/291) patients who presented with seizures preoperatively, 27% (54/197) were seizure free, whereas 73% (143/197) continued to experience seizures after surgery. Postoperatively, 63% (183/291) patients developed seizures, 22% (40/183) of whom did not present with seizures prior to surgery. Among 32% (94/291) of patients without seizures prior to surgery, 43% (40/94) developed seizures and 57% (54/94) remained seizure free after surgery. Forty-one percent (120/291) of patients developed intractable seizures during the course of their disease. Among patients who were on AEDs (239/291; 82%), 60% were on monotherapy, 27% were on 2, 11% were on 3, and 2% were on 4 or more AEDs. The most commonly used AED was levetiracetam, followed by phenytoin and valproic acid.
Factors Influencing Seizures in Low-Grade Glioma Patients
Rates of intractable seizures differed by WHO 2007 classification histological subtype (P = .048) (Table 2), with the observed rate highest for the oligoastrocytoma histological subtype (51%; 38/74), and observed rates for other subtypes as follows: oligodendroglioma (41%; 56/137), astrocytoma (28%; 17/61), and LGG not otherwise specified (NOS) (47%; 9/19). However, based on the WHO 2016 classification, the observed rates for intractable seizures were similar: 45% (41/92) in oligodendroglioma, IDH mutated, 1p/19q codeleted; 44% (48/109) in diffuse astrocytoma, IDH mutated, 1p/19q intact; and 40% (21/53) in astrocytoma, IDH wild-type grade II LGG. There was also no significant difference in seizure frequency during the preoperative and postoperative periods on the basis of the WHO 2016 classification (Table 3), although a trend toward higher seizure presentation prior to surgery was observed in patients with IDH-mutated LGG (P = .09). On univariate analysis, male sex was significantly associated with higher seizure incidence both during the preoperative [123/158 (78%) vs 74/133 (56%), P < .001] and postoperative periods [113/158 (72%) vs 70/133 (53%), P < .001]; men were also more likely to develop intractable seizures [76/158 (48%) vs 44/133 (33%), P = .01] (Table 4). Extent of surgical resection was not associated with postoperative seizure incidence [gross total resection 22/41 (54%); subtotal resection 84/128 (66%); biopsy 76/120 (63%); P = .38]. Multivariate analysis was not performed because only one variable was significantly associated with preoperative and postoperative seizure risk, whereas sex and histologic classification were the only 2 variables significantly associated with intractable seizures on univariate analysis.
Table 2.
Seizure Frequency Based on Histologic Tumor Classification (World Health Organization 2007)
| Seizure | Oligodendroglioma | Oligoastrocytoma | Astrocytoma | NOS | P |
|---|---|---|---|---|---|
| Preoperative (n = 197) | 98/137 (72%) | 50/74 (68%) | 37/61 (61%) | 12/19 (63%) | .48 |
| Postoperative (n = 183) | 90/137 (66%) | 50/74 (68%) | 31/61 (51%) | 12/19 (63%) | .17 |
| Intractable seizure (n = 120) | 56/137 (41%) | 38/74 (51%) | 17/61 (28%) | 9/19 (47%) | .048 |
Abbreviation: NOS, not otherwise specified.
Value in bold is statistically significant.
Table 3.
Seizure Frequency Based on Integrated Tumor Classification (World Health Organization 2016)
| Seizure | Oligodendroglioma, IDH-mutated, 1p/19q codeleted | Diffuse astrocytoma, IDH-mutated, 1p/19q intact | Diffuse astrocytoma, IDH wild-type | NOS | P |
|---|---|---|---|---|---|
| Preoperative (n = 197) | 67/92 (73%) | 75/109 (69%) | 31/53 (58%) | 24/37 (65%) | .34 |
| Postoperative (n = 183) | 57/92 (62%) | 75/109 (69%) | 30/53 (57%) | 21/37 (57%) | .37 |
| Intractable seizure (n = 120) | 41/92 (45%) | 48/109 (44%) | 21/53 (40%) | 10/37 (27%) | .27 |
Abbreviations: IDH, isocitrate dehydrogenase; NOS, not otherwise specified.
Table 4.
Univariate Analysis of Patient Characteristics and Seizures
| Factors | Preop seizure | Postop seizures | Intractable seizures |
|---|---|---|---|
| Sex | <.001 | <.001 | .01 |
| Age (< or ≥ 45), y | .65 | .34 | .90 |
| Tumor location (temporal vs nontemporal) | .49 | .95 | .70 |
| Laterality | .94 | .64 | .77 |
| IDH mutation status | .09 | .14 | .70 |
| Histologic classification, WHO 2007 | .48 | .17 | .048 |
| Integrated classification, WHO 2016 | .34 | .37 | .27 |
| Surgery type | NA | .38 | NA |
Abbreviations: IDH, isocitrate dehydrogenase; NA, not applicable; NOS, not otherwise specified; Postop, postoperative; Preop, preoperative; WHO, World Health Organization.
Value in bold is statistically significant.
Seizure and its Relation to Survival in Low-Grade Glioma Patients
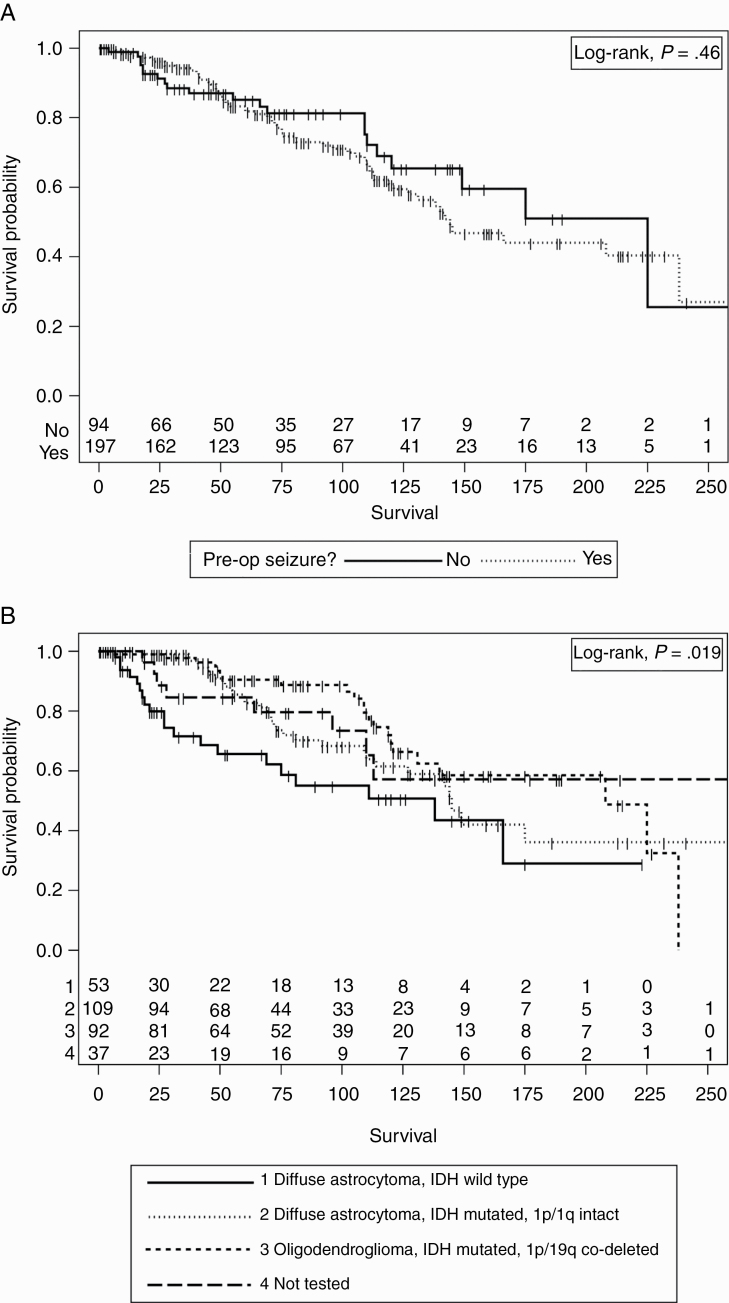
The median survival of our patients was 166 months (95% CI, 138-238 months). The survival of patients who presented with seizures did not significantly differ from those who did not have seizures prior to diagnosis of LGG (log-rank test, P = .46) (Figure 1A). As expected, survival differences were detected based on the WHO 2016 classification (log-rank test, P = .019) (Figure 1B).
Figure 1.
A, Survival vs seizure presentation. There was no significant difference in survival between patients who presented with a preoperative (pre-op) seizure and those who did not. B, Survival vs World Health Organization (WHO) 2016 integrated glioma classification. Significant differences were detected based on the WHO 2016 classification (1 = diffuse astrocytoma, isocitrate dehydrogenase [IDH] wild-type, grade II, 2 = diffuse astrocytoma, IDH-mutated, 1p/19q intact, grade II, 3 = oligodendroglioma, IDH-mutated, 1p/19q codeleted, grade II, 4 = not tested).
Discussion
LGG in adults is usually associated with seizure occurrence either as presenting symptoms or at recurrence and can adversely affect the patient’s quality of life. The mechanism of tumor epileptogenesis is complex, and specific risk factors for seizures in patients with LGG are not fully understood. In this retrospective study, we analyzed a series of 291 patients with LGG and identified clinical, histopathological, and molecular factors predictive of early and recurrent seizures.
Consistent with historical data, the majority of our patients presented with seizures prior to diagnosis of LGG and less than half experienced uncontrolled seizures. New-onset seizure as a presenting symptom of LGG is reported in 70% to 90% of patients, with approximately 15% to 50% of patients with LGG demonstrating pharmacoresistant seizures throughout the course of their disease.2,5,6,14–17 In our study, oligodendroglioma was the most common histological subtype based on the WHO 2007 classification, followed by oligoastrocytoma and astrocytoma, which is in accordance with other reports.15 After reclassification based on the WHO 2016 classification, the majority of our patients harbored IDH-mutant tumors, with a similar number of patients classified as oligodendroglioma, IDH-mutated, 1p19q-codeleted, and diffuse astrocytoma, IDH-mutated, noncodeleted 1p/19q, also in keeping with prior reports.15,18 The distribution of seizure semiology in our study is in agreement with other major studies, with generalized-onset seizures in 38% to 54%, focal-onset seizures in 20% to 40%, and focal onset with secondary generalization seizures in 10% to 70%.8,9,14–16 However, we acknowledge the challenges in categorization of focal-onset with secondary generalization and generalized-onset seizures in patients with brain tumors. Further, it is presumed that LGG patients with generalized seizures have focal onset.
Histologic and Molecular Factors Associated With Seizures
Based on the WHO 2007 classification, oligoastrocytoma was most likely to be associated with intractable seizures compared to oligodendroglioma, astrocytoma, and NOS subtypes. In previous studies, oligodendroglioma has been reported to be the most common histologic type of epilepsy-related LGG.8,9 This is believed to be due its propensity for the cortical regions of the brain, compared to astrocytoma, which tends to be situated in subcortical white matter.5,8,9,17 Tumor anatomical and functional locations have been previously reported to be predictive of epilepsy incidence, suggesting that glioma-related seizures may be triggered by interactions between the glioma and the neocortex.2,19 In contrast, a recent large case-control study demonstrated that patients with astrocytoma had significantly more recurrent seizures than patients with other histologic subtypes.16,20 However, the significant association of histologic subtypes and epilepsy diminish after incorporation of molecular parameters. After reclassification based on the 2016 WHO classification, we found no significant impact of LGG subtypes on seizure occurrence. Our finding is similar to a recent large, observational, retrospective multicenter study involving 1509 LGG patients and a recent smaller retrospective report including 63 LGG patients in which molecular markers (IDH mutation and 1p/19q codeletion status) did not predict seizure development or outcome.2,15 Conversely, a better seizure outcome was observed in patients in an IDH1/2-mutated and 1p/19q codeleted subgroup in a recent retrospective study with 155 diffuse LGG patients.21 A significant association between IDH mutation and preoperative seizures in diffuse gliomas has been previously demonstrated. IDH-mutant glioma cells reduce α-ketoglutarate to D-2-hydroxyglutarate, which is structurally similar to glutamate and may mimic the activity of glutamate in NMDA receptors, potentially leading to seizures.6,10,11 In line with this emerging hypothesis, a trend toward higher incidence of seizure presentation in patients with IDH-mutated LGG was observed in our study.
These data, including our study, suggest that glioma-related epilepsy may be more complex than molecular classification alone, and clinical factors such as tumor location (both the lobe of the brain and cortex vs subcortex), sex, extent of surgery, and more all contribute to seizure occurrence and frequency.2 Further, our study suggests a difference in the impact between molecular and histological classifications on the risk of seizure in LGG patients, emphasizing the importance of reclassification of LGG patients according to the WHO 2016 classification.
Clinical Factors Associated With Seizures
An intriguing finding in our study is the significant correlation of male sex with the risk of developing seizures at presentation, after surgery, and intractable seizures throughout the disease course. A large multicenter French study including 1509 patients with LGG also identified male sex as one of the independent predictors for tumor-related epileptic seizures at diagnosis and was significantly associated with shorter malignant progression-free survival and overall survival.2 However, the association between sex and epilepsy in patients with gliomas is not well defined.
Sex differences in glioma biology and outcome have been previously described but remain poorly understood. Retrospectively acquired lower-grade glioma transcriptome data from The Cancer Genome Atlas including 285 male and 228 female patients demonstrated a sex difference in glucose metabolism in glioma with sex-specific effects on outcome.22 Male patients with high glycolytic gene expression had significantly decreased median overall survival relative to male low-glycolytic patients; no such differences were seen in the female patient groups. Mutations in IDH were also observed to interact with the glycolytic phenotype. Among male patients with IDH-mutated LGG, those with high glycolytic gene expression had a lower median survival compared to men with low-glycolytic gene expression; this difference was also not observed in female patients with IDH mutation. For male patients with IDH wild-type LGG, the median survival for high- and low-glycolytic patients were similar. These data provide a possible link between male sex in LGG and possible metabolism-modulating activity of IDH mutation. With the reported higher frequency of seizures in IDH-mutant LGG patients due to the resemblance of 2-HG to glutamate, it is tempting to hypothesize that a difference of glycolytic phenotype predisposes a subset of male IDH-mutant LGG patients to glioma-associated epilepsy. This previously uncharacterized finding warrants further investigation.
Factors that were previously reported to have a significant association with seizure occurrence in LGG were also analyzed in our study. We found no significant association between epilepsy and age, tumor location, laterality, and surgery type in patients with LGG. Age 45 years or older has previously been identified as an independent predictor of favorable seizure control after oncological treatment and the postoperative period.2,7 We dichotomized our patients’ age using a similar cutoff value (age < 45 and ≥ 45 years) but did not find similar results. Some studies have reported temporal or insular location as an unfavorable factor for seizure occurrence and control.2,9,17 However, our findings are similar to other studies reporting no significant correlation between tumor location and seizure outcome.7,8 The inconsistencies among several studies including our data may be attributable to variability in categorization of location (per individual lobes vs temporal and nontemporal) and a small study population. Similar to other reports, none of our patients with LGG in subcortical locations experienced seizures at presentation.9 Gross total resection of LGG has been reported to be associated with favorable seizure control in several studies.2,7,9,15,20,23,24 Additionally, improvement both in the proportion of seizure-free patients and the durability of seizure freedom were observed in patients, with EOR of greater than 80%.21,23 However, we failed to find a similar association in our study population. Thirty-three percent to 72% of patients in these previously mentioned studies underwent gross total resection, and a median EOR of 90% was achieved in one retrospective volumetric study.7,9,15,20,23,24 Only 14% of our patients underwent gross total resection or lobectomy, which may account for our lack of association between EOR and postoperative seizure control. Nevertheless, a study including 1509 LGG patients showed significant associations between total resection and subtotal resection and seizure control, despite only 9% of the study population achieving gross total resection.2 This suggests that our small study population is another potential reason for the lack of significant association in our study. In addition, the timing of surgery from the initial clinical presentation was not recorded in our study. In other studies, surgical intervention showed a strong tendency to predict better seizure outcome.17,24
Seizure and Survival
Other studies have reported that seizures at presentation may correlate with improved survival in LGG patients.2,6,9,24 The mechanism of this potential correlation is believed to be multifactorial, related to earlier recognition of tumor (detection bias), the slow growth of LGG, as well as association with IDH mutation and seizures.6,14,19 However, we did not find a significant survival benefit based on history of seizure at presentation in our study.
Our study is one of the few studies that investigated the influence of the WHO 2016 classification of glioma and seizure in LGG; 15,21 compared to the study by Robert et al15 and Ius and colleagues,21 our study has the largest number of patients with molecular testing and subsequent reclassification based on the WHO 2016 classification (n = 254 vs n = 63 and n = 155, respectively). Our study highlights the inconsistencies of available data and the complexity of glioma-related epilepsy. A prospective, larger-population study of LGG patients diagnosed according to the up-to-date WHO 2016 classification is important to better understand and identify the links between epilepsy and LGG, thus allowing an individualized treatment approach based on the patient’s risk factors.
Our study has several limitations, including retrospective analysis and some missing molecular data in a subset of patients. The lack of statistical significance of multiple factors on univariate analysis may reflect reduced statistical power from a moderately sized study population. Our study did not address the influence of tumor volume, radiation therapy, and chemotherapy on seizure control, as well as the association between uncontrolled seizure and tumor recurrence. Owing to the limitation of the retrospective nature of this study, the widely used Engel Epilepsy Surgical Outcome Scale was not used to define the seizure outcome after surgery.25
Conclusion
Our study confirms that seizures are common in patients with LGG prior to diagnosis, and almost half of patients develop intractable seizures throughout their disease course. A trend in higher preoperative seizure occurrence in IDH-mutated LGG patients is noted, consistent with previous data. Contrary to most data, age, temporal location, and the updated WHO 2016 integrated classification did not predict seizure development or outcome in our series. We describe an unanticipated association between male sex and seizure risk in LGG patients found in one other study2 warranting further investigation on the possible molecular bases of sex differences in the epileptogenesis in LGG. A larger, multicenter prospective study is essential to identify clinical factors and histopathologic and molecular markers that can predict seizure risk in patients with LGG. Determination of possible links between histopathologic and molecular markers and epilepsy remains a vital importance in the effective management of epilepsy in LGG patients.
Funding
None declared.
Conflict of interest statement. None declared.
References
- 1. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 2. Pallud J, Audureau E, Blonski M, et al. . Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(pt 2):449–462. [DOI] [PubMed] [Google Scholar]
- 3. Maschio M, Sperati F, Dinapoli L, et al. . Weight of epilepsy in brain tumor patients. J Neurooncol. 2014;118(2):385–393. [DOI] [PubMed] [Google Scholar]
- 4. Klein M, Engelberts NH, van der Ploeg HM, et al. . Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–520. [DOI] [PubMed] [Google Scholar]
- 5. Ruda R, Bello L, Duffau H, Soffietti R.. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan X, Li Y, Shan X, et al. . Seizures at presentation are correlated with better survival outcomes in adult diffuse glioma: a systematic review and meta-analysis. Seizure. 2018;59:16–23. [DOI] [PubMed] [Google Scholar]
- 7. Shan X, Fan X, Liu X, et al. . Clinical characteristics associated with postoperative seizures control in adult low-grade gliomas: a systematic review and meta-analysis. Neuro Oncol. 2018;20(3): 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Chi XS, Hu X, et al. . Predictors and mechanisms of epilepsy occurrence in cerebral gliomas: what to look for in clinicopathology. Exp Mol Pathol. 2017;102(1):115–122. [DOI] [PubMed] [Google Scholar]
- 9. Chang EF, Potts MB, Keles GE, et al. . Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Judkins J, Thomas C, et al. . Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liubinas SV, D’Abaco GM, Moffat BM, et al. . IDH1 mutation is associated with seizures and protoplasmic subtype in patients with low-grade gliomas. Epilepsia. 2014;55(9):1438–1443. [DOI] [PubMed] [Google Scholar]
- 12. Kwan P, Arzimanoglou A, Berg AT, et al. . Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. [DOI] [PubMed] [Google Scholar]
- 13. Fisher RS, Cross JH, French JA, et al. . Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. [DOI] [PubMed] [Google Scholar]
- 14. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts M, Northmore T, Shires J, Taylor P, Hayhurst C. Diffuse low grade glioma after the 2016 WHO update, seizure characteristics, imaging correlates and outcomes. Clin Neurol Neurosurg. 2018;175:9–15. [DOI] [PubMed] [Google Scholar]
- 16. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(suppl 9):12–17. [DOI] [PubMed] [Google Scholar]
- 17. Brogna C, Gil Robles S, Duffau H. Brain tumors and epilepsy. Expert Rev Neurother. 2008;8(6):941–955. [DOI] [PubMed] [Google Scholar]
- 18. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 19. Pallud J, McKhann GM. Diffuse low-grade glioma-related epilepsy. Neurosurg Clin N Am. 2019;30(1):43–54. [DOI] [PubMed] [Google Scholar]
- 20. Berntsson SG, Merrell RT, Amirian ES, et al. . Glioma-related seizures in relation to histopathological subtypes: a report from the Glioma International Case-Control Study. J Neurol. 2018;265(6):1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ius T, Pauletto G, Tomasino B, et al. . Predictors of postoperative seizure outcome in low grade glioma: from volumetric analysis to molecular stratification. Cancers (Basel). 2020;12(2):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ippolito JE, Yim AK, Luo J, Chinnaiyan P, Rubin JB. Sexual dimorphism in glioma glycolysis underlies sex difference in survival. JCI Insight. 2017;2(15):e92142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu DS, Al-Wala A, Mehalechko C, et al. . An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J Neurosurg. 2018;128(4):1084–1090. [DOI] [PubMed] [Google Scholar]
- 24. Capelle L, Fontaine D, Mandonnet E, et al. ; French Réseau d’Étude des Gliomes . Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 25. Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:609–621. [Google Scholar]