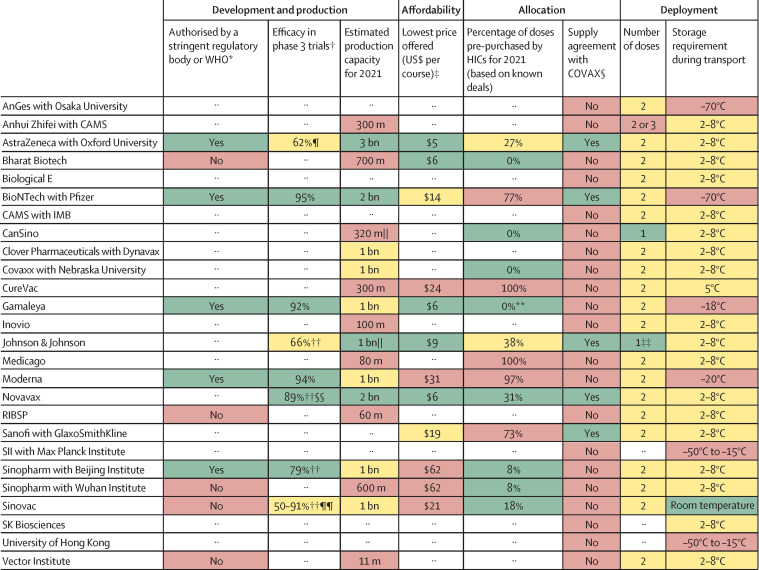
Figure 2.
Key characteristics of leading vaccine candidates with traffic-light system signalling potential for achieving global vaccine immunity
The sources and methodology are documented in appendix 1, including the criteria for assigning a green, amber, or red light for each characteristic. Candidates shown in this figure have been approved or authorised on an emergency basis for human use in one or more countries, are in phase 3 clinical testing, or are under contract with CEPI or the COVAX Facility, as of Feb 3, 2021. Where there are no entries, either the data are unavailable or it is too early to know (eg, for vaccines in the early stages of development). Both Institut Pasteur (in collaboration with Merck) and the University of Queensland were developing COVID-19 vaccine candidates with funding from CEPI, but these clinical trials have been discontinued. CAMS=Chinese Academy of Medical Sciences. CEPI=Coalition for Epidemic Preparedness Innovations. HIC=high-income country. IMB=Institute of Medical Biology (China). RIBSP=Research Institute for Biological Safety Problems (Kazakhstan). SII=Serum Institute of India. *Only for vaccines that have been approved or granted emergency authorisation by at least one regulatory body; WHO publishes a list of stringent regulatory authorities,2 and can itself grant emergency use listing or prequalification for vaccines. †Clinical trial designs, including efficacy endpoints, differed for the various vaccine candidates; the efficacy figures might therefore not be perfectly comparable. Some of these results are interim analyses from phase 3 studies. Due to the emergence of new variants of the virus, the conditions under which trials take place vary, and not all vaccines are tested against the same variants. ‡These prices are the lowest the developers offered to any country or purchasing bloc; median prices for a range of countries are presented in figure 3. §The COVAX Facility has first right of refusal for a potential combined total of more than 1 billion doses in 2021 of vaccine candidates being developed by CEPI-funded companies: Biological E, Clover Pharmaceuticals, CureVac, Inovio, Moderna, Novavax, Oxford University/AstraZeneca, SK Biosciences, and the University of Hong Kong.3 ¶This was the result in the main efficacy analysis for participants receiving two standard doses, as specified in the protocol. The result in the out-of-protocol arm (a half dose followed by a standard dose) was 90%. This first-generation vaccine might offer less protection against a strain of SARS-CoV-2 first identified in South Africa. ||For the assignment of risk levels, we treated a single dose of a one-dose vaccine as equivalent to two doses of a two-dose vaccine. **One HIC (Hungary) has purchased 2 million doses, corresponding to 0·4% of all purchased doses; due to rounding, the figure presented in the dashboard is 0%. ††These interim phase 3 results have not been published in peer-reviewed journals; the figures were sourced from press releases by companies or researchers running the clinical trials. ‡‡The developer is also testing a two-dose version. §§This was the efficacy reported from a phase 3 trial in the UK; Novavax reported a lower efficacy level in a smaller phase 2b clinical trial in South Africa (49%). These results have not yet been published in peer-reviewed journals. ¶¶Sinovac and its research partners have reported a range of efficacy levels on the basis of phase 3 trials in Brazil (50%), Indonesia (65%), Turkey (91%), and the United Arab Emirates (86%), but none of these results have been published in peer-reviewed journals.