Figure 1.
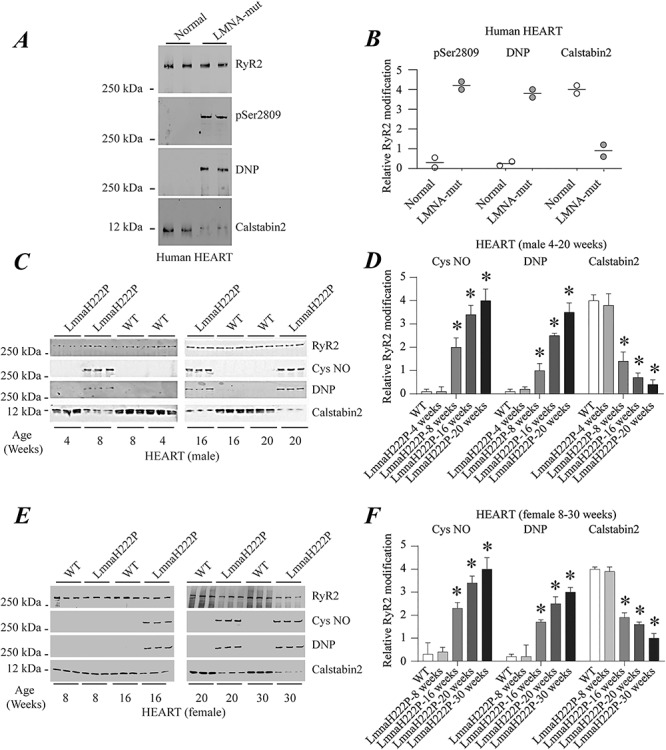
RyR2 remodeling in the hearts of human subjects with cardiomyopathy and LMNA mutations and LmnaH222P/H222P mice. (A) Immunoblot showing RyR2 immunoprecipitated from protein extracts of human hearts and PKA-catalyzed phosphorylation (pSer2809), oxidation (DNP) and calstabin2 depletion. The two samples at the left are from normal controls and the two samples at the right are from human subjects with LMNA mutation and cardiomyopathy. (B) Quantifications of results from scanning immunoblots (values normalized to RyR2). Individual values are shown (n = 2). (C) Immunoblots showing RyR2 immunoprecipitated from protein extracts of male WT and LmnaH222P/H222P (LmmaH222P) mouse hearts and S-nitrosylation (Cys NO), channel oxidation (DNP) and calstabin2 depletion. Three samples from LmnaH222P and WT mice are shown for each time point. (D) Quantifications of results from scanning immunoblots (values normalized to RyR2). Data are means ± SEM (n = 3); *P < 0.05 WT versus LmnaH222P at 8, 16 and 20 weeks of age. (E) Immunoblots showing RyR2 immunoprecipitated from protein extracts of female WT and LmnaH222P/H222P (LmnaH222P) mouse hearts and S-nitrosylation (Cys NO), oxidation (DNP) and calstabin2 depletion. Three samples from LmnaH222P and WT mice are shown for each time point. (F) Quantifications of results from scanning immunoblots (values normalized to RyR2). Data are means ± SEM (n = 3); *P < 0.05 WT versus LmnaH222P at 16, 20 and 30 weeks of age. Migrations of molecular mass standards are indicated to the left of the blots in (A, C andE).
