Abstract
Root canal debridement, which includes the removal of infected tissues and microbial biofilms, is considered the corner stone of root canal treatment. Chemical adjuncts play a multitude of functions in this regard, as tissue solvents, antimicrobial agents and for removing the smear layer. These adjuncts (irrigants) are usually delivered using a syringe and needle. With increasing knowledge of the complexity of root canal anatomy and tenacity of microbial biofilms, the need for strategies that potentiate the action of these irrigants within the root canal system cannot be overemphasized. Several such activated irrigation strategies exist. The aim of this review is to comprehensively discuss the different irrigant activation methods from the context of clinical studies.
Keywords: Sodium hypochlorite, Microbial reduction, Pain, Root canal treatment, Sonic, Ultrasonic
KEY CHALLENGES IN ROOT CANAL TREATMENT
The aim of chemo-mechanical root canal debridement is to remove microbial biofilms, vital and/or necrotic pulp tissue and hard tissue debris generated during instrumentation [1]. Sodium hypochlorite (NaOCl; 0.5%–6%), the most commonly used irrigant, is a non-specific proteolytic agent which dissolves pulp tissue, demonstrates antimicrobial and antibiofilm effects, but it is unable to remove any accumulated hard tissue debris. Hence, its use is often followed by a demineralizing/chelating agent, typically, ethylenediaminetetraacetic acid (EDTA; 10%–17%) [2,3,4].
Instrumentation combined with needle-and-syringe irrigation of NaOCl has been shown to reduce the microbial load from root canals using culture-based approaches [5,6]. However, the increasing evidence on the complexity of the root canal anatomy highlighted the challenges in optimal disinfection of the root canal system [7,8,9]. Both manual and engine-driven instrumentation systems are unable to contact 100% of the root canal wall, implying that, untouched walls retain pulp remnants and biofilms, contributing to post-treatment disease [10]. Where the walls are touched, i.e., scrubbed and/or shaved mechanically, a smear layer is created, and no chelating agent can completely remove it [11,12].
The role of microorganisms in root canal infections has long been established [13,14,15]. Biofilms are spatio-temporally organized, adherent masses of microorganisms, encapsulated in their self-produced extracellular matrix [16,17]. Endodontic bacterial biofilms are concentrated within the main canal, and the anatomic eccentricities outlined above. Even in the absence of bacteria, the biofilm matrix alone can result in chronic inflammation [18], indicating that antimicrobial strategies used in endodontics should result in disruption of the biofilm architecture.
Therefore, the role of irrigation in achieving optimal debridement of the canals cannot be overemphasized. Traditionally, irrigants are delivered into the canal with a syringe and a needle. However, the presence of an apical “vapor lock”, i.e., air bubble entrapment, has been shown to impede optimal irrigant exchange throughout the root canal system with syringe and needle (positive pressure) irrigation, contributing markedly to poor canal debridement [19]. This phenomenon has been demonstrated both in vitro and in vivo [20], yet it remains unclear if it has a direct impact on clinical outcomes. Thus, the key challenges in root canal debridement include i) root canal anatomy, ii) biofilm nature of infection, and iii) insufficiencies in contemporary instrumentation and irrigation [21,22].
Activated irrigation is a potentially important method to counteract these problems, with an aim of chemically and mechanically activating irrigants to improve their antimicrobial and tissue-dissolving efficiency and to enhance their penetration into the complex root canal anatomy by displacing air bubbles [23,24,25].
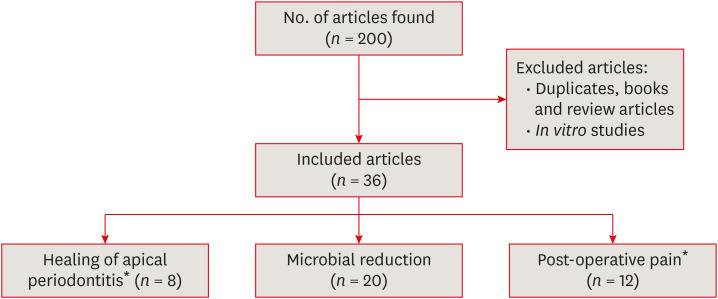
The goal of root canal treatment is prevention or treatment of apical periodontitis, which can be studied using primary outcome measures (healing of periradicular lesions) or surrogate outcome measures (e.g. microbial reduction). In addition, postoperative pain is an important patient-centered clinical outcome measure. The aim of this paper is to review the effects of different irrigant activation methods and their effects on several selected clinical outcome measures (periradicular healing, microbial reduction and post-operative pain). Literature search was performed on PubMed up to September 2019, using keywords as listed in Table 1 and article selections as described in Figure 1.
Table 1. Electronic search strategy with PubMed.
| Number | Search strategy | Results |
|---|---|---|
| #1 | root canal | 36,454 |
| #2 | agitation OR activation OR machine-assisted OR syringe irrigation OR manual dynamic agitation OR sonic OR ultrasonic OR light activated disinfection OR photodynamic therapy OR photo activated disinfection OR laser OR photon-induced photo acoustic streaming OR apical negative pressure OR multisonic | 5,608,203 |
| #3 | microbial reduction OR antimicrobial OR biofilm OR healing OR apical periodontitis OR pain OR quality of life | 3,191,671 |
| #4 | #1 AND #2 AND #3 | 200 |
Figure 1. A flowchart of the article selection process.
*Four studies investigated the effects on 2 outcome measures.
ACTIVATED IRRIGATION STRATEGIES
There appears to be no consensus on the use of terminology pertaining to irrigant activation and agitation. Hence, this review will include various published methods of irrigant activation and agitation. Manual dynamic agitation (MDA) uses a well-fitting gutta-percha master cone in 2–3 mm up-and-down strokes to improve the displacement and exchange of the solution [26]. This simple and cost-effective method has been found to be more effective than static needle-and-syringe irrigation [27]. Brushes are used in a manner similar to MDA described above to agitate the irrigant solution. Canalbrush (Coltène Whaledent, Langenau, Germany) was shown to have significantly better debridement of the root canal walls compared to MDA [28]. Instruments (rotary and oscillation) such as the XP-endo Finisher (XPF; FKG Dentaire SA, La Chaux-de-Fonds, Switzerland), Self-adjusting File (SAF; ReDent Nova, Ra'anana, Israel), Finisher GF Brush (MedicNRG, Kibbutz Afikim, Israel) and Finishing file (Engineered Endodontics, Menomonee Falls, WI, USA) have also been introduced for activated irrigation. While the SAF is a cleaning-shaping-irrigation system [29], the other instruments listed above are used supplementary to root canal preparation.
Sonics and ultrasonics are used at a frequency of 1–6 kHz and 25–30 kHz, respectively, to vibrate an instrument to generate flow and shear stresses within the fluid [30,31]. Ultrasonics are based on the principles of acoustic streaming (a phenomenon generated in a fluid field consisted of eddy flows) and cavitation (a phenomenon when bubbles are generated in the liquid that implodes due to tremendous force), creating pressure-vacuum effect [32,33], although there is substantial uncertainty in the literature if the latter may be produced by ultrasonic root canal instruments [31]. The terms ultrasonically activated irrigation and passive ultrasonic irrigation are used interchangeably [34]. Another, relatively new, strategy is multisonics (e.g., GentleWave System, Sonendo, Mission Viejo, CA, USA), which generates multiple frequency sound waves to optimize fluid dynamics and irrigant exchange. The resulting hydrodynamic cavitation with the implosion of microbubbles has been claimed to enhance disinfection [35,36].
Coherent and non-coherent light has been suggested for antimicrobial treatment of root canals. Light-activated disinfection (antimicrobial photodynamic therapy [aPDT], or photoactivated disinfection), targets specific microbial cells using a non-toxic photosensitizer dye and a light source with specific wavelengths [37]. Common photosensitizers include methylene blue, toluidine blue, Rose Bengal and indocyanine green.
Coherent light (laser) sources used for disinfection include Erbium:Yttrium-Aluminum-Garnet (Er:YAG); Erbium, Chromium:Yttrium-Scandium-Gallium-Garnet (Er,Cr:YSGG); Neodymium:Yttrium-Aluminum-Garnet (Nd:YAG); potassium titanyl phosphate (KTP); diode and carbon dioxide (CO2). In laser-activated irrigation, a laser beam interacts with the tissues by absorption, converting it to thermal energy. The degree of absorption is influenced by the wavelength used and composition of the tissue, e.g., water or hydroxyapatite. While most lasers designed for application inside the root canal come with radial firing tips, the erbium family of laser (Er:YAG) works on the principle of photon induced photoacoustic streaming (PIPS), which uses short laser pulses at the entrance of the root canal, with continuous irrigation.
The above-mentioned methods rely on irrigant delivery using positive-pressure delivery, except the multisonic system which has been claimed to generate negative pressure [38]. Another exclusively negative-pressure device for irrigant delivery is commercially known as EndoVac (Kavo Kerr, Brea, CA, USA), where a set of cannulas are used in the depth of the canal to literally suck the irrigant out of the canal [39]. The main advantage of this system has been claimed to be the ability to allow irrigant exchange without periradicular extrusion [40].
CLINICAL EFFICACY OF ACTIVATED IRRIGATION
While numerous studies have been performed in the laboratory (using in vitro and/or ex vivo models), to demonstrate the (lack of) effectiveness of activated irrigation strategies, their inclusion into a routine clinical protocol should be based on the highest level of evidence, i.e., randomized controlled clinical trials and systematic reviews of clinical trials. Three key clinical outcome measures are commonly studied in endodontics: healing of apical periodontitis, antimicrobial effectiveness and post-operative pain.
HEALING OF APICAL PERIODONTITIS
Healing outcomes have been reported in endodontics using imaging techniques (2D and/or 3D) or clinical symptomatology, or both. The periapical index is one of the most widely used methods, using a scale of 5 scores, ranging from 1 (healthy) and 5 (severe periodontitis) for 2D radiographic examinations [41]. A modified version has been adopted for assessment of periapical status by 3D imaging [42]. Clinically, the teeth are examined for any abnormalities related to periodontal pockets, mobility, swelling, sinus tract or abscess, discomfort/tenderness on percussion or palpation, including patient's reporting of pain related to the treated tooth. A summary of the pertinent articles was summarized in Table 2.
Table 2. Summary of the methodology and results of studies for healing of apical periodontitis (8 studies total).
| Study | Year | Experimental groups & irrigants used | Outcome measures | Key findings | |
|---|---|---|---|---|---|
| Liang et al. [43] | 2013 | ⋄ Syringe needle irrigation (3 × 2 mL 5.25% NaOCl with 10 sec in canal without agitation; final irrigation time 60 sec) | ⋄ Presence of radiographic periapical lesion | ⋄ No significant difference between ultrasonically activated irrigation and syringe irrigation in periapical healing. | |
| ⋄ Ultrasonically activated irrigation (3 × 2mL 5.25% NaOCl with 10 sec of activation; final irrigation time 60 sec) | ⋄ Periapical radiograph and CBCT (lesion area and volume) | ||||
| ▪ Absence | |||||
| ▪ Reduction of radiolucency | |||||
| ▪ Enlargement of radiolucency | |||||
| ▪ Uncertain | |||||
| Tang et al. [44] | 2015 | ⋄ Ultrasonically activated irrigation (2.5% NaOCl) | ⋄ Presence of radiographic periapical lesion | ⋄ No significant difference between all groups in healing after 6 months and 12 months. | |
| ⋄ Ultrasonically activated irrigation (silver ion antibacterial solution) | ⋄ Periapical radiograph (PAI score 1–5) | ||||
| ⋄ Syringe needle irrigation (2.5% NaOCl) | |||||
| Cohenca et al. [45] | 2015 | ⋄ Apical negative pressure (30 sec 5.25% NaOCl, 30 sec 17% EDTA, 30 sec 5.25% NaOCl) | ⋄ Dog teeth, experimentally induced periapical lesions | ⋄ All groups had similar periapical response. | |
| ⋄ Ultrasonically activated irrigation (30 sec 5.25% NaOCl + 20 sec of activation; 30 sec 17% EDTA + 20 sec of activation; 30 sec 5.25% NaOCl + 20 sec of activation) | ⋄ Presence of radiographic periapical lesion | ⋄ Apical negative pressure group had the mildest infiltration of inflammatory cells. | |||
| ⋄ Syringe needle irrigation (30 sec of 5.25% NaOCl, 30 sec of 17% EDTA, 30 sec of 5.25% NaOCl) | ⋄ Periapical radiograph (lesion area) | ||||
| ⋄ Histology (conventional & fluorescence microscopy & staining) | |||||
| ▪ Thickness of PDL: score 1–4 | |||||
| ▪ Inflammatory infiltration: score 1–4 | |||||
| ▪ Resorption process of the mineralized tissue: score 1–2 (presence or absence) | |||||
| De Jesus et al. [46] | 2019 | ⋄ Apical negative pressure (30 sec 5.25% NaOCl; 30 sec 17% EDTA; 30 sec 5.25% NaOCl) | ⋄ Dog teeth, experimentally induced periapical lesions | ⋄ No significant difference between the groups; repair of apical periodontitis occurred in up to 60% of cases regardless of irrigation protocol used. | |
| ⋄ Ultrasonically activated irrigation (30 sec 5.25% NaOCl + 20 sec of activation; 30 sec 17% EDTA + 20 sec of activation; 30 sec 5.25% NaOCl + 20 sec of activation) | ⋄ Presence of radiographic periapical lesion | ||||
| ⋄ Syringe needle irrigation (30 sec of 5.25% NaOCl; 30 sec of 17% EDTA; 30 sec of 5.25% NaOCl) | ⋄ Periapical radiograph (PAI score 1–5) | ||||
| ⋄ Immunohistochemistry | |||||
| ▪ Tumor necrosis factor (TNF-α) | |||||
| ▪ Osteopontin (OPN) | |||||
| ▪ Interleukin 1α (IL-1α) | |||||
| Sigurdsson et al. [47] | 2016 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Presence of radiographic periapical lesion | ⋄ GentleWave resulted in 97.4% success rate of healing. | |
| ⋄ Periapical radiograph (PAI score 1–5) | |||||
| Sigurdsson et al. [48] | 2016 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Presence of radiographic periapical lesion | ⋄ GentleWave resulted in 97.3% success rate of healing. | |
| ⋄ Periapical radiograph (PAI score 1–5) | |||||
| Sigurdsson et al. [49] | 2018 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Presence of radiographic periapical lesion | ⋄ GentleWave resulted in 97.7% success rate of healing. | |
| ⋄ Periapical radiograph (PAI score 1–5) | ⋄ 43 out of 44 were completely functional. | ||||
| Martins et al. [50] | 2013 | ⋄ Syringe needle irrigation + Ca(OH)2 (1st appt: 5 mL 3% NaOCl during instrumentation, Ca(OH)2 dressing; 2nd appt: 5 mL 3% NaOCl) | ⋄ Presence of radiographic periapical lesion | ⋄ No significant differences in periapical healing between the groups. | |
| ⋄ Er,Cr:YSGG (1st appt: 2 mL saline during instrumentation, 4 times irradiation with 2 with canals filled with distilled water, 2 in dry condition; 2nd appt: repeat irradiation procedures, 5 mL saline rinse 1 min) | ⋄ Periapical radiograph (PAI score 1–5) | ||||
NaOCl, sodium hypochlorite; CBCT, cone beam computed tomography; EDTA, ethylenediaminetetraacetic acid; PAI, periapical index; Er,Cr:YSGG, Erbium, Chromium doped Yttrium Scandium Gallium Garnet.
Ultrasonic irrigation
One study [43] identified no significant differences between ultrasonic irrigation (95.1%) and syringe irrigation (88.4%) in periapical healing, using cone beam computed tomography and periapical radiography, 10–19 months after treatments. The total final irrigation time and volume were standardized to 1 minute and 6 mL, respectively. Comparing patient-reported outcomes (masticatory function and discomfort), symptoms (tenderness on percussion) and radiographic analysis, Tan and coworkers [44] reported no significant differences in any of these parameters between ultrasonic activation of 2.5% NaOCl or a silver ion antibacterial solution, and needle-and-syringe (manual) irrigation with 2.5% NaOCl.
Two other studies [45,46] compared the effect of ultrasonic activation, negative pressure irrigation and manual irrigation on the healing of experimentally-induced periapical lesions in dogs. The NaOCl-EDTA-NaOCl protocol was followed in both the studies, with all solutions activated for 20 seconds with an endosonic file in the ultrasonic group. Outcomes were evaluated using radiography, histology and immunohistochemistry. Results from these studies showed no significant difference in radiographic healing between the irrigation groups 180 days post-treatment.
Multisonic irrigation
Sigurdsson et al. [47,48,49] evaluated the healing following the use of GentleWave system in a single-arm randomized clinical trial. All the samples were treated with the manufacturer's recommended protocol (3% NaOCl-distilled water rinse-8% EDTA-distilled water-8% EDTA-distilled water rinse). In these studies, teeth with all kinds of pulpal and periapical diagnosis were included in the samples. Considering both healed and healing lesions as successful (favorable) outcomes, the success rate was reported to be consistently high (97.4% and 97.3% respectively) over the 6-and 12-month period [47,48].
Laser-activated irrigation
Comparing saline irrigation activated by Er,Cr:YSGG laser, versus a combination of syringe irrigation of NaOCl with calcium hydroxide dressing, Martins et al. [50] reported similar healing results for the two groups. One potential problem with this study was the difference in irrigating solutions (saline vs. 3% hypochlorite) between the groups, which cast doubts to the validity of the conclusion.
MICROBIAL REDUCTION
One key aim of root canal preparation is to reduce the microbial load to a threshold at which the body can manage with its immune response [51]. However, this threshold remains unknown. In practice, therefore, reduction of the microbial content from the root canal system to the best of the clinician's ability is imperative. Antimicrobial efficacy in vivo has been investigated by traditional culture-based, as well as molecular techniques such as quantitative real-time polymerase chain reaction (qRT-PCR) [52]. A summary of the pertinent articles was summarized in Table 3.
Table 3. Summary of the methodology and results of studies for microbial reduction (20 studies total).
| Study | Year | Experimental groups & irrigants used | Outcome measures | Key findings |
|---|---|---|---|---|
| Huffaker et al. [53] | 2010 | ⋄ Syringe needle irrigation (2 × NaOCl with 30 sec in canal without agitation; final irrigation time 60 sec) | ⋄ Bacterial sampling and culturing (CFU) | ⋄ No significant difference in the ability of sonic and needle control group to eliminate cultivable bacteria from root canals. |
| ⋄ Sonic irrigation (2 × NaOCl with 30 sec of activation; final irrigation time 60 sec) | ||||
| Rico-Romano et al. [54] | 2016 | ⋄ Sonic irrigation (30 sec 5.25% NaOCl) | ⋄ Bacterial sampling and culturing (CFU) | ⋄ No significant differences between NaOCl and CHX groups. |
| ⋄ Ultrasonically activated irrigation (1 min 5.25% NaOCl) | ⋄ Effectiveness of ultrasonic activation was significantly higher than sonic activation. | |||
| ⋄ Sonic irrigation (30 sec 2% CHX) | ||||
| ⋄ Ultrasonically activated irrigation (1 min 2% CHX) | ||||
| Beus et al. [55] | 2012 | ⋄ Syringe needle irrigation (6 mL 1% NaOCl) | ⋄ Bacterial sampling and culturing | ⋄ No significant differences between syringe needle irrigation and ultrasonically activated irrigation. |
| ⋄ Ultrasonically activated irrigation (2 × 30 sec 1% NaOCl of activation; 2 × 30 sec 17% EDTA of activation; 2 × 30 sec 2% CHX of activation) | ||||
| Cohenca et al. [56] | 2013 | ⋄ Apical negative pressure (30 sec 5.25% NaOCl; 30 sec 17% EDTA; 30 sec 5.25% NaOCl; final irrigation time 90 sec) | ⋄ Dog teeth, experimentally induced periapical lesions | ⋄ Apical negative pressure was significantly better in reducing gram (−) bacteria than syringe needle irrigation. |
| ⋄ Ultrasonically activated irrigation (10 sec 5.25% NaOCl + 20 sec activation; 10 sec 17% EDTA + 20 sec activation; 10 sec 5.25% NaOCl + 20 sec activation; final irrigation time 90 sec) | ⋄ Bacterial sampling and culturing (CFU) | ⋄ No statistically significant differences between syringe needle irrigation and ultrasonically activated irrigation. | ||
| ⋄ Syringe needle irrigation (30 sec 5.25% NaOCl; 30 sec 17% EDTA; 30 sec 5.25% NaOCl; final irrigation time 90 sec) | ||||
| ⋄ [Positive control] syringe needle irrigation (3 × 30 sec saline) | ||||
| ⋄ [Negative control] no inoculation of bacteria | ||||
| Nakamura et al. [57] | 2018 | ⋄ Ultrasonically activated irrigation (2 × 30 sec 2 mL 2.5% NaOCl of activation; 2 × 30 sec 2 mL 17% EDTA of activation; 2 × 30 sec 2 mL 2.5% NaOCl of activation) | ⋄ Human teeth with necrotic pulps and asymptomatic apical periodontitis. | ⋄ Ultrasonic activation was more effective than syringe needle irrigation for reducing the number of bacteria but not the endotoxin levels in root canals of teeth with apical periodontitis. |
| ⋄ Syringe needle irrigation (2 × 30 sec 2 mL 2.5% NaOCl; 2 × 30 sec 2 mL 17% EDTA; 2 × 30 sec 2 mL 2.5% NaOCl) | ⋄ Bacterial sampling (total bacteria count qPCR and endotoxin levels by limulus amebocyte lysate essay) | |||
| Carver et al. [59] | 2007 | ⋄ Hand/rotary technique (15 mL 6% NaOCl syringe needle irrigation) | ⋄ Bacterial sampling and culturing (CFU) | ⋄ Additional one minute of ultrasonic activation resulted in significant reduction in bacteria count. |
| ⋄ Hand/rotary/ultrasonic technique (15 mL 6% NaOCl syringe needle irrigation; 1 min 6% NaOCl of activation [15 mL /min flow rate]) | ||||
| Paiva et al. [60] | 2013 | ⋄ Before instrumentation | ⋄ Human teeth with necrotic pulp | ⋄ Ultrasonic activated irrigation did not have significant enhancement in disinfection beyond instrumentation based on this small sample. |
| ⋄ After instrumentation (syringe needle irrigation of 2.5% NaOCl; 17% EDTA; 2.5% NaOCl) | ⋄ Bacterial sampling (qPCR) | |||
| ⋄ Ultrasonic activated irrigation (1 min 2.5% NaOCl; needle irrigation of 3 mL 2.5% NaOCl) | ||||
| Paiva et al. [61] | 2012 | ⋄ Ultrasonic activated irrigation (1 min 2 mL 2.5% NaOCl; needle irrigation of 3 mL 2.5% NaOCl) | ⋄ Human teeth with necrotic pulps and apical periodontitis. | ⋄ No significant difference between additional irrigation methods with ultrasonic or chlorhexidine rinse. |
| ⋄ Chlorhexidine rinse (needle irrigation of 5 mL 2% CHX) | ⋄ Bacterial sampling and culturing (qPCR) | ⋄ Supplementary disinfection with either ultrasonic activated irrigation or chlorhexidine rinse reduced bacterial count. | ||
| Burleson et al. [62] | 2007 | ⋄ Hand/rotary technique (15 mL 6% NaOCl syringe needle irrigation; [15 mL/min flow rate]) | ⋄ Human teeth with necrotic pulps and apical periodontitis. | ⋄ Canal and isthmus cleanliness values (biofilm and necrotic debris) were significantly higher for hand/rotary/ultrasound technique at all levels evaluated. |
| ⋄ Hand/rotary/ultrasonic technique (15 mL 6% NaOCl syringe needle irrigation; 1 min 6% NaOCl of ultrasonic activation [15 mL/min flow rate]) | ⋄ Extracted after irrigation protocol; histology | |||
| ⋄ [Negative control] no treatment | ||||
| Gutarts et al. [63] | 2005 | ⋄ Hand/rotary technique (15 mL 6% NaOCl syringe needle irrigation; [15 mL/min flow rate]) | ⋄ Human teeth with necrotic pulps and apical periodontitis. | ⋄ Canal and isthmus cleanliness values (remaining pulp tissues) were significantly higher for hand/rotary/ultrasound technique at all levels except one. |
| ⋄ Hand/rotary/ultrasonic technique (15 mL 6% NaOCl syringe needle irrigation; 1 min 6% NaOCl of ultrasonic activation [15 mL/min flow rate]) | ⋄ Extracted after irrigation protocol; histology | |||
| ⋄ [Negative control] no treatment | ||||
| Pawar et al. [64] | 2012 | ⋄ Apical negative pressure (40 mL 0.5% NaOCl) | ⋄ Human teeth with necrotic pulps and apical periodontitis. | ⋄ Antimicrobial efficacy of apical pressure irrigation was comparable with syringe needle irrigation. |
| ⋄ Syringe needle irrigation (40 mL 0.5% NaOCl) | ⋄ Bacterial sampling and culturing | |||
| Cohenca et al. [65] | 2010 | ⋄ Apical negative pressure (10 mL 2.5% NaOCl; canal filled with no apical negative pressure for 60 sec; needle irrigation of sterile saline) | ⋄ Dog teeth, experimentally induced periapical lesions | ⋄ No significant difference between bacterial reduction between apical negative pressure and syringe needle irrigation with triantibiotic dressing. |
| ⋄ Syringe needle irrigation + triantibiotic dressing (10 mL 2.5% NaOCl; canal filled for 60 sec; sterile saline; triantibiotic dressing; 10 mL sterile saline) | ⋄ Bacterial sampling and culturing (CFU) | |||
| Lindström et al. [66] | 2017 | ⋄ Nd:YAG (4 times irradiation with canals filled with saline [20 sec intervals between each application]) | ⋄ Human teeth with apical periodontitis. | ⋄ Nd:YAG laser irradiation did not significantly produce negative bacterial culture compared to syringe needle irrigation. |
| ⋄ Syringe needle irrigation (1% NaOCl; 2 min 3 mL 15% EDTA; 30 mL 1% NaOCl; final irrigation volume 30 mL of 1% NaOCl) | ⋄ Bacterial sampling and culturing | |||
| Pourhajibagher et al. [67] | 2017 | ⋄ After removal of root filling | ⋄ Human teeth with secondary endodontic infections | ⋄ TBO-mediated PAD was significantly effective in reducing bacterial activity in secondary persistent endodontic infection. |
| ⋄ PAD (30 sec irradiation with toluidine blue O) | ⋄ Bacterial sampling and culturing; analytical profile index assays; 16S ribosomal RNA gene sequencing | |||
| Pourhajibagher et al. [68] | 2018 | ⋄ PAD (60 sec irradiation with toluidine blue O) | ⋄ Human teeth with apical periodontitis. | ⋄ TBO-mediated PAD was significantly effective in reducing bacterial activity in infected roots. |
| ⋄ Bacterial sampling and culturing (multiplex real-time PCR) | ||||
| Pourhajibagher et al. [69] | 2018 | ⋄ PAD (60 sec irradiation with toluidine blue O) | ⋄ Human teeth with apical periodontitis. | ⋄ TBO-mediated PAD significantly decreased microbial diversity and count of infected roots. |
| ⋄ Bacterial sampling and culturing (PCR) | ||||
| Asnaashari et al. [70] | 2017 | ⋄ PAD (60 sec irradiation with 0.5 mL of toluidine blue O) | ⋄ Human teeth with apical periodontitis requiring root canal retreatment. | ⋄ PAD was more effective in reduction of Enterococcus faecalis in infected root canals when compared to calcium hydroxide group. |
| ⋄ Ca(OH)2 (2 weeks post-instrumentation) | ⋄ Bacterial sampling and culturing (CFU) | |||
| Jurič et al. [71] | 2014 | ⋄ After removal of root filling | ⋄ Human teeth with apical periodontitis requiring root canal retreatment. | ⋄ Endodontic retreatment alone produced a significant reduction in number of bacterial species. |
| ⋄ After instrumentation and irrigation (needle irrigation of 1 mL 2.5% NaOCl; 1 min 1 mL 17% EDTA; 1 mL 2.5% NaOCl) | ⋄ Bacterial sampling and culturing (CFU) | ⋄ Combination of retreatment procedures with aPDT was statistically more effective. | ||
| ⋄ aPDT (60 sec irradiation with phenothiazinium chloride) | ||||
| Garcez et al. [72] | 2010 | ⋄ After accessing the root canal | ⋄ Human teeth with apical periodontitis requiring root canal retreatment. | ⋄ The addition of aPDT to root canal treatment led to further major reduction in bacterial load. |
| ⋄ After instrumentation and irrigation (needle irrigation of 5 mL 17% EDTA; 5 mL PBS) | ⋄ Bacterial sampling and culturing | |||
| ⋄ aPDT (60 sec irradiation with polyethylenimine chlorin[e6]) | ||||
| López et al. [73] | 2015 | ⋄ SX 400 ppm Sterilox +PAD (120 sec irradiation with toluidine blue O) | ⋄ Dog teeth, experimentally induced periapical lesions | ⋄ PAD did not produce significant differences in the scores for apical inflammation when used after chemo-mechanical preparation |
| ⋄ 2% NaOCl + PAD (120 sec irradiation with toluidine blue O) | ⋄ Light microscopy (severity of inflammation) | |||
| ⋄ 5% NaOCl + PAD (120 sec irradiation with toluidine blue O) | ||||
| ⋄ Saline + PAD (120 sec irradiation with toluidine blue O) | ||||
| ⋄ SX 400 ppm Sterilox | ||||
| ⋄ 2% NaOCl | ||||
| ⋄ 5% NaOCl | ||||
| ⋄ Saline |
NaOCl, sodium hypochlorite; CFU, colony-forming unit; CHX, chlorhexidine; EDTA, ethylenediaminetetraacetic acid; qPCR, quantitative polymerase chain reaction; Nd:YAG, Neodymium-doped Yttrium Aluminum Garnet; PAD, photoactivated disinfection; TBO, toluidine blue O; aPDT, antimicrobial photodynamic therapy; PBS, phosphate-buffered saline.
Sonic and ultrasonic irrigation
While sonic agitation of the irrigant has been shown to demonstrate similar microbial reduction compared to manual irrigation [53], sonic activation was reported as significantly less effective than ultrasonic activation, regardless of the irrigant used (NaOCl or CHX) [54]. Comparing ultrasonic with syringe irrigation, 2 studies [55,56] demonstrated no significant differences in microbial counts between the two groups in humans and dogs respectively. On the other hand, Nakamura et al. [57] showed that ultrasonic reduced significantly more bacteria than syringe irrigation using a molecular microbiological approach. The differences between these studies may be attributed to two reasons: differences in the irrigation protocol and in the analytical methods. While Cohenca et al. [56] and Nakamura et al. [57] used sequential irrigation with NaOCl-EDTA-NaOCl, Beus et al. [55] used a NaOCl-EDTA-CHX sequence with an activation cycle for each of the irrigants, and included a frequent replenishment cycle. Furthermore, irrigant concentrations and duration of activation were different between those studies. Both Beus et al. [55] and Cohenca et al. [56] used a culture-based approach. Contemporary microbiological studies demonstrate that several microbes may be viable but not cultivable (VBNC) [17,58], resulting in false-negative results. This may be mitigated by molecular approaches [52].
Ultrasonic irrigation was tested as a supplementary step following chemo-mechanical preparation and manual irrigation in 5 ex vivo studies [59,60,61,62,63], all of which used a 1 minute ultrasonication of NaOCl after completing the canal instrumentation. While one study [59] showed that ultrasonic activation significantly improved disinfection, another [60] showed conflicting results. One key difference between these studies was the analytical method: one [59] used a culture-based approach, while the other [60] used a molecular approach. Paiva et al. [61] compared a final rinse of CHX with ultrasonically activated NaOCl using microbial culture and end-point PCR, and reported no significant differences in microbial reduction between the groups. Burleson et al. [62] and Gutarts et al. [63] showed that ultrasonic activation significantly improved the elimination of tissue debris and microbial biofilms from root canals and isthmi, compared to needle-and-syringe irrigation.
Apical negative-pressure irrigation
Microbial reduction from root canals following apical negative pressure irrigation was reported in several studies [56,64,65], 2 of which [56,65] were performed in experimentally-induced periradicular lesions in dogs. Apical negative pressure irrigation was shown to be similar in antimicrobial effectiveness compared to ultrasonics, but was more effective than manual irrigation [56]. However, when a supplementary step of intracanal medication (tri-antibiotic paste) was included, manual irrigation was as effective as apical negative pressure in reducing microbial loads [65]. On the other hand, Pawar et al. [64] showed no significant difference between apical negative pressure irrigation and traditional needle-and-syringe irrigation.
Laser-activated irrigation (LAI)
Only one study explored the antibacterial effects of Nd:YAG laser + saline, with needle-and-syringe irrigation with NaOCl (1%) and EDTA (15%) [66]. Microbiological culturing showed no significant difference between the groups. It is unknown if the use of NaOCl activated by Nd:YAG laser would have resulted in different extent of microbial reduction.
aPDT
Different photosensitizer dyes such as toluidine blue, phenothiazinium chloride, and polyethylenimine chlorin(e6) have been used with diode laser for disinfection. Bacterial diversity and quantity were significantly less in groups treated with aPDT using toluidine blue as photosensitizer in primary and secondary root canal infections [67,68,69]. aPDT was more effective than calcium hydroxide treatment for 2 weeks in reducing microbial counts [70], suggesting that aPDT could be included as a single-visit retreatment protocol.
Other photosensitizers such as phenothiazinium chloride and polyethylenimine and chlorin(e6), activated with a diode laser of 660 nm wavelength appear to significantly reduce bacterial loads compared to the control group (i.e., no aPDT) [71,72]. Only one study reported that PDT did not significantly reduce microbial loads compared to the use of antiseptics (Sterilox or NaOCl) or saline. However, this study did not use any photosensitizer, but attempted to activate the antiseptics with light [73].
POST-OPERATIVE PAIN
Pain following treatment is measured subjectively using the visual analog scale (VAS) from a scale of 0 to 10 or 100, with 10 or 100 being the most severe pain respectively. Furthermore, the post-operative use of analgesics is also used as an outcome measure to indicate post-operative pain. One recent systematic review and meta-analysis [74] reported that machine-assisted agitation (ultrasonic, sonic and negative pressure irrigation) demonstrated less post-operative pain than syringe irrigation. A summary of the pertinent articles was summarized in Table 4.
Table 4. Summary of the methodology and results of studies for post-operative pain (12 studies total).
| Study | Year | Experimental groups & irrigants used | Outcome measures | Key findings |
|---|---|---|---|---|
| Ramamoorthi et al. [75] | 2015 | ⋄ Syringe needle irrigation (40 sec 4 mL 3% NaOCl; flow rate 0.1 mL/s−1) | ⋄ Patients with symptomatic irreversible pulpitis | ⋄ Sonic irrigation resulted in significantly less post-operative pain and analgesics intake than syringe needle irrigation group. |
| ⋄ Sonic irrigation (2 × 1 min 2 mL 3% NaOCl of activation) | ⋄ VAS score (0–10) at 8, 24 & 48 hours | |||
| ⋄ Amount of analgesic intake | ||||
| Topçuoğlu et al. [76] | 2018 | ⋄ Syringe needle irrigation (1 min 5 mL 3% NaOCl; 1 min 2 mL 17% EDTA) | ⋄ Patients with symptomatic irreversible pulpitis | ⋄ Manual dynamic agitation caused greater post-operative pain after root canal treatment of symptomatic irreversible pulpitis cases in the first 24 hours. |
| ⋄ Sonic irrigation (1 min 5 mL 3% NaOCl of activation; 1 min 2 mL 17% EDTA of activation) | ⋄ VAS score (0–10) at 6, 24, 48 & 72 hours and 1 week | |||
| ⋄ Ultrasonic activated irrigation (1 min 5 mL 3% NaOCl of activation; 1 min 2 mL 17% EDTA of activation) | ||||
| ⋄ Manual dynamic agitation (1 min 5 mL 3% NaOCl of activation; 1 min 2 mL 17% EDTA of activation) | ||||
| Al-Zaka [77] | 2012 | ⋄ Syringe needle irrigation (30 sec 1 mL 2.5% NaOCl; 1 mL 2.5% NaOCl) | ⋄ Patients with asymptomatic irreversible pulpitis | ⋄ Apical negative irrigation significantly lower post-operative pain compared to syringe needle irrigation and sonic irrigation at all intervals evaluated. |
| ⋄ Sonic irrigation (30 sec 1 mL 2.5% NaOCl of activation; needle irrigation of 1 mL 2.5% NaOCl) | ⋄ VAS score (1–4) at 4, 24 & 48 hours | |||
| ⋄ Apical negative pressure (30 sec 1 mL 2.5% NaOCl; needle irrigation of 1 mL 2.5% NaOCl) | ||||
| Yilmaz et al. [78] | 2019 | ⋄ Syringe needle irrigation (1 min 4 mL 2.5% NaOCl) | ⋄ Patients with nonvital pulps | ⋄ Significant less post-operative pain was reported when sonic irrigation was used compared to other groups. |
| ⋄ Sonic irrigation (1 min 4 mL 2.5% NaOCl of activation) | ⋄ VAS score (0–10) at 8, 24, 48 & 72 hours | ⋄ The combinations of sonic irrigation with NaOCl and QMix had the most significant decrease in pain. | ||
| ⋄ Syringe needle irrigation (1 min 4 mL 2.5% NaOCl; 3 mL sterile water; 1 min 3 mL QMix) | ⋄ Amount of analgesic intake | |||
| ⋄ Sonic irrigation (1 min 4 mL 2.5% NaOCl of activation; 3 mL sterile water; 1 min 3 mL QMix of activation) | ||||
| Tang et al. [44] | 2015 | ⋄ Ultrasonically activated irrigation (2.5% NaOCl) | ⋄ Patients with chronic apical periodontitis | ⋄ The post-operative pain levels were significantly less in ultrasonically activated irrigation groups when compared to syringe needle irrigation group. |
| ⋄ Ultrasonically activated irrigation (silver ion antibacterial solution) | ⋄ VAS score (0–10) at 24 hours | |||
| ⋄ Syringe needle irrigation (2.5% NaOCl) | ||||
| Middha et al. [79] | 2017 | ⋄ Continuous ultrasonic irrigation (15 mL 5.25% NaOCl) | ⋄ Patients with nonvital pulps and apical periodontitis | ⋄ Pain was significantly lower in the continuous ultrasonic irrigation group when compared to the syringe needle irrigation group only on first day post-operatively. |
| ⋄ Syringe needle irrigation (15 mL 5.25% NaOCl) | ⋄ VAS score (0–10) at every day for 7 days | |||
| ⋄ Amount of analgesic intake | ||||
| Coelho et al. [80] | 2019 | ⋄ aPDT (3 min irradiation with methylene blue) | ⋄ Patients with necrotic pulps | ⋄ Photodynamic therapy had significant effect in decreasing post-operative pain at 24 and 72 hours intervals. |
| ⋄ No aPDT (3 min no irradiation with methylene blue) | ⋄ VAS score (0–10) at 24 & 72 hours and 1 week | |||
| Gondim et al. [81] | 2010 | ⋄ Syringe needle irrigation (2.5% NaOCl; final irrigation volume 130 mL of NaOCl inclusive of instrumentation after each instrument) | ⋄ Patients with asymptomatic irreversible pulpitis or normal pulp | ⋄ Between 0–4 hours and 4–24 hours, intake of analgesics was significantly less in group treated by apical negative pressure irrigation. |
| ⋄ Apical negative pressure (2.5% NaOCl; final irrigation volume 130 mL of NaOCl inclusive of instrumentation after each instrument) | ⋄ VAS score (0–10) at 4, 24 & 48 hours | ⋄ The use of apical negative pressure irrigation can result in significant reduction in post-operative pain. | ||
| ⋄ Amount of analgesic intake | ||||
| Topçuoğlu et al. [82] | 2018 | ⋄ Syringe needle irrigation (20 mL 2.5% NaOCl [instrumentation]; 5 mL 17% EDTA; 5 mL distilled water) | ⋄ Patients with symptomatic irreversible pulpitis | ⋄ More post-operative pain was reported at the 6-, 24- and 48-hour intervals in syringe needle irrigation group when compared with apical negative pressure group. |
| ⋄ Apical negative pressure (20 mL 2.5% NaOCl [instrumentation]; 5 cycles of irrigation of 2.5% NaOCl; final irrigation time 30 sec; 5 mL 17% EDTA, 5 mL distilled water) | ⋄ VAS score (0–10) at 6, 24, 48 & 72 hours and 1 week | |||
| Sigurdsson et al. [47] | 2016 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Patients with tooth indicated for root canal treatment | ⋄ 3% of patients experienced moderate pain (VAS 7–8) within 2 days after initial treatment. |
| ⋄ VAS score (0–10) at 2, 7, 14 days and each review visit | ||||
| Sigurdsson et al. [48] | 2016 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Patients with tooth indicated for root canal treatment | ⋄ 3.8% of patients experienced moderate pain (VAS 7–8) within 2 days. |
| ⋄ VAS score (0–10) at 2, 7, 14 days and every 3, 6 and 12 months | ⋄ No pain reported at 2 weeks, 6 months and 12 months. | |||
| Sigurdsson et al. [49] | 2018 | ⋄ GentleWave (3% NaOCl, distilled water rinse; 8% EDTA; 30 sec final distilled water rinse; 2 min 8% EDTA; 15 sec distilled water rinse) | ⋄ Patients with tooth indicated for root canal treatment | ⋄ No patients experienced moderate to severe post-operative pain at 2-, 4- and 7-days post-operatively. |
| ⋄ VAS score (0–10) at before treatment, 2, 7, 14 days and each review visit |
NaOCl, sodium hypochlorite; VAS, visual analogue scale; EDTA, ethylenediaminetetraacetic acid; aPDT, antimicrobial photodynamic therapy.
Sonic irrigation
Sonic irrigation was found to cause significantly less post-operative pain compared to needle irrigation at 8, 24 and 48 hours. Patients who were treated with manual irrigation consumed significantly more analgesics at 0 to 24 hours [75]. However, in that study, sonic irrigation was used for a longer time than syringe irrigation. Other studies [76,77] made an effort to standardize the irrigant volume and duration, with results showing no significant difference between manual and sonic irrigation, although the latter resulted in significantly less pain than MDA and negative pressure irrigation. Sonics with QMix (an irrigant mixture of CHX and EDTA) resulted in significantly less pain than sonic or needle-and-syringe irrigation with 2.5% NaOCl [78].
Ultrasonic irrigation
Ultrasonic irrigation was shown to result in significantly less post-operative pain, compared to syringe irrigation, regardless of the irrigating solution (2.5% NaOCl or silver ions) [44]. One study [76] reported no significant difference in pain levels between syringe irrigation, sonic and PUI up to 1 week after treatment, while MDA resulted in significantly more pain, up to 24 hours post-treatment. When continuous ultrasonic was used, post-operative pain scores were significantly less than syringe irrigation for 24 hours after treatment, after which pain scores did not differ significantly between the groups [79].
aPDT
The only study [80] which evaluated post-operative pain after aPDT reported that supplementary (3 minutes) disinfection with aPDT (methylene blue PS) resulted in significantly less pain scores compared to syringe irrigation. The authors indirectly attributed this to more effective microbial reduction in the aPDT-treated groups although this outcome measure was not investigated.
Apical negative pressure irrigation
Negative pressure irrigation was shown to result in significantly less post-operative pain compared to needle irrigation [77,81,82] and sonic irrigation [77]. However, analgesic intake varied between the studies, with Gondim et al. [81] reporting significantly less intake in the negative pressure irrigation group. That was in contrast to Topçuoğlu et al. [82] who showed no difference between the groups.
Multisonics
The literature on post-operative pain using multisonics (GentleWave system) were single-arm studies with no comparative group. Post-operative pain was reported to be minimal when GentleWave was used, with > 3.8% of patients reporting post-operative pain within 2 days [47,48,49]. As those studies were done in the same center, it would be nice if there should be further reports from another independent site of investigation.
CONCLUSIONS
The contemporary knowledge of root canal anatomy and microbiology highlighted the need to develop irrigation strategies that can optimally disinfect the root canal system. However, the lack of standardized study designs (both in vitro and in vivo) precludes drawing conclusions for clinical recommendations. Despite the limitations with microbiological studies, the presence of cultivable bacteria prior to root canal obturation has a negative impact on outcomes [83]. Whether activated irrigation strategies will render root canals bacteria-free or will significantly improve healing outcomes, remain unknown. Well-controlled randomized controlled clinical trials are required to draw clinically relevant conclusions.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Cheung AWT, Cheung GSP.
- Data curation: Cheung AWT.
- Formal analysis: Cheung AWT, Lee AHC.
- Writing - original draft: Cheung AWT.
- Writing - review & editing: Cheung GSP, Lee AHC.
References
- 1.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Neelakantan P, Cheng CQ, Mohanraj R, Sriraman P, Subbarao C, Sharma S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro . Int Endod J. 2015;48:602–610. doi: 10.1111/iej.12354. [DOI] [PubMed] [Google Scholar]
- 5.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–328. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 6.Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–312. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 7.Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol. 1984;58:589–599. doi: 10.1016/0030-4220(84)90085-9. [DOI] [PubMed] [Google Scholar]
- 8.Gulabivala K, Aung TH, Alavi A, Ng YL. Root and canal morphology of Burmese mandibular molars. Int Endod J. 2001;34:359–370. doi: 10.1046/j.1365-2591.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 9.Martins JNR, Marques D, Silva EJNL, Caramês J, Versiani MA. Prevalence studies on root canal anatomy using cone-beam computed tomographic imaging: a systematic review. J Endod. 2019;45:372–386.e4. doi: 10.1016/j.joen.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira JF, Jr, Pérez AR, Marceliano-Alves MF, Provenzano JC, Silva SG, Pires FR, Vieira GCS, Rôças IN, Alves FRF. What happens to unprepared root canal walls: a correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int Endod J. 2018;51:501–508. doi: 10.1111/iej.12753. [DOI] [PubMed] [Google Scholar]
- 11.Ballal NV, Jain I, Tay FR. Evaluation of the smear layer removal and decalcification effect of QMix, maleic acid and EDTA on root canal dentine. J Dent. 2016;51:62–68. doi: 10.1016/j.jdent.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Machado R, Garcia LDFR, da Silva Neto UX, Cruz Filho AMD, Silva RG, Vansan LP. Evaluation of 17% EDTA and 10% citric acid in smear layer removal and tubular dentin sealer penetration. Microsc Res Tech. 2018;81:275–282. doi: 10.1002/jemt.22976. [DOI] [PubMed] [Google Scholar]
- 13.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 14.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–484. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 15.Sundqvist G. Bacteriological studies of necrotic dental pulps [dissertation] Umeå, Sweden: Umeå University; 1976. [Google Scholar]
- 16.Neelakantan P, Romero M, Vera J, Daood U, Khan AU, Yan A, Cheung GSP. Biofilms in endodontics-current status and future directions. Int J Mol Sci. 2017;18:1748. doi: 10.3390/ijms18081748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali IAA, Cheung BPK, Yau JYY, Matinlinna JP, Lévesque CM, Belibasakis GN, Neelakantan P. The influence of substrate surface conditioning and biofilm age on the composition of Enterococcus faecalis biofilms. Int Endod J. 2020;53:53–61. doi: 10.1111/iej.13202. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez T, Shrestha A, Kishen A. Inflammatory potential of monospecies biofilm matrix components. Int Endod J. 2019;52:1020–1027. doi: 10.1111/iej.13093. [DOI] [PubMed] [Google Scholar]
- 19.Tay FR, Gu LS, Schoeffel GJ, Wimmer C, Susin L, Zhang K, Arun SN, Kim J, Looney SW, Pashley DH. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J Endod. 2010;36:745–750. doi: 10.1016/j.joen.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera J, Arias A, Romero M. Dynamic movement of intracanal gas bubbles during cleaning and shaping procedures: the effect of maintaining apical patency on their presence in the middle and cervical thirds of human root canals-an in vivo study. J Endod. 2012;38:200–203. doi: 10.1016/j.joen.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221–230. doi: 10.1046/j.1365-2591.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu MK, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J. 2001;34:137–141. doi: 10.1046/j.1365-2591.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 23.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Neelakantan P, Devaraj S, Jagannathan N. Histologic assessment of debridement of the root canal isthmus of mandibular molars by irrigant activation techniques ex vivo . J Endod. 2016;42:1268–1272. doi: 10.1016/j.joen.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Dioguardi M, Di Gioia G, Illuzzi G, Ciavarella D, Laneve E, Troiano G, Lo Muzio L. Passive ultrasonic irrigation efficacy in the vapor lock removal: systematic review and meta-analysis. Sci World J. 2019;2019:6765349. doi: 10.1155/2019/6765349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machtou P. Irrigation investigation in endodontics [master's thesis] Paris, France: Paris VII University; 1980. [Google Scholar]
- 27.McGill S, Gulabivala K, Mordan N, Ng YL. The efficacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen ‘bio-molecular film’ from an ex vivo model. Int Endod J. 2008;41:602–608. doi: 10.1111/j.1365-2591.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 28.Al-Ali M, Sathorn C, Parashos P. Root canal debridement efficacy of different final irrigation protocols. Int Endod J. 2012;45:898–906. doi: 10.1111/j.1365-2591.2012.02046.x. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira JF, Jr, Alves FR, Almeida BM, de Oliveira JC, Rôças IN. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod. 2010;36:1860–1865. doi: 10.1016/j.joen.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Martin H. Ultrasonic disinfection of the root canal. Oral Surg Oral Med Oral Pathol. 1976;42:92–99. doi: 10.1016/0030-4220(76)90035-9. [DOI] [PubMed] [Google Scholar]
- 31.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415–426. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad M, Pitt Ford TJ, Crum LA. Ultrasonic debridement of root canals: acoustic streaming and its possible role. J Endod. 1987;13:490–499. doi: 10.1016/s0099-2399(87)80016-x. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad M, Pitt Ford TR, Crum LA, Walton AJ. Ultrasonic debridement of root canals: acoustic cavitation and its relevance. J Endod. 1988;14:486–493. doi: 10.1016/S0099-2399(88)80105-5. [DOI] [PubMed] [Google Scholar]
- 34.Căpută PE, Retsas A, Kuijk L, Chávez de Paz LE, Boutsioukis C. Ultrasonic irrigant activation during root canal treatment: a systematic review. J Endod. 2019;45:31–44.e13. doi: 10.1016/j.joen.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of root canal debridement of human molars using the GentleWave System. J Endod. 2015;41:1701–1705. doi: 10.1016/j.joen.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Haapasalo M, Shen Y, Wang Z, Park E, Curtis A, Patel P, Vandrangi P. Apical pressure created during irrigation with the GentleWave™ system compared to conventional syringe irrigation. Clin Oral Investig. 2016;20:1525–1534. doi: 10.1007/s00784-015-1632-z. [DOI] [PubMed] [Google Scholar]
- 37.Ali IAA, Neelakantan P. Light activated disinfection in root canal treatment- a focused review. Dent J (Basel) 2018;6:31. doi: 10.3390/dj6030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charara K, Friedman S, Sherman A, Kishen A, Malkhassian G, Khakpour M, Basrani B. Assessment of apical extrusion during root canal irrigation with the novel GentleWave system in a simulated apical environment. J Endod. 2016;42:135–139. doi: 10.1016/j.joen.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Versiani MA, Alves FR, Andrade-Junior CV, Marceliano-Alves MF, Provenzano JC, Rôças IN, Sousa-Neto MD, Siqueira JF., Jr Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. Int Endod J. 2016;49:1079–1087. doi: 10.1111/iej.12559. [DOI] [PubMed] [Google Scholar]
- 40.Romualdo PC, de Oliveira KMH, Nemezio MA, Küchler EC, Silva RAB, Nelson-Filho P, Silva LAB. Does apical negative pressure prevent the apical extrusion of debris and irrigant compared with conventional irrigation? A systematic review and meta-analysis. Aust Endod J. 2017;43:129–137. doi: 10.1111/aej.12162. [DOI] [PubMed] [Google Scholar]
- 41.Ørstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2:20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 42.Estrela C, Bueno MR, Azevedo BC, Azevedo JR, Pécora JD. A new periapical index based on cone beam computed tomography. J Endod. 2008;34:1325–1331. doi: 10.1016/j.joen.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Liang YH, Jiang LM, Jiang L, Chen XB, Liu YY, Tian FC, Bao XD, Gao XJ, Versluis M, Wu MK, van der Sluis L. Radiographic healing after a root canal treatment performed in single-rooted teeth with and without ultrasonic activation of the irrigant: a randomized controlled trial. J Endod. 2013;39:1218–1225. doi: 10.1016/j.joen.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Tang Z, Wang H, Jiang S. Clinical study of single-visit root canal treatment with a nickel-titanium (Ni-Ti) rotary instrument combined with different ultrasonic irrigation solutions for elderly patients with chronic apical periodontitis. Biomed Mater Eng. 2015;26(Supplement 1):S311–S318. doi: 10.3233/BME-151318. [DOI] [PubMed] [Google Scholar]
- 45.Cohenca N, Romualdo PC, da Silva LA, da Silva RA, de Queiroz AM, De Rossi A, Nelson-Filho P. Tissue response to root canal irrigation systems in dogs' teeth with apical periodontitis. Clin Oral Investig. 2015;19:1147–1156. doi: 10.1007/s00784-014-1340-0. [DOI] [PubMed] [Google Scholar]
- 46.Jesus SF, Cohenca N, Romualdo PC, Nelson-Filho P, Queiroz AM, Sousa-Neto MD, Paula-Silva FWG, Silva LABD. Radiographic and immunohistochemical evaluation of root canal treatment using different irrigation systems. Braz Dent J. 2019;30:123–132. doi: 10.1590/0103-6440201901702. [DOI] [PubMed] [Google Scholar]
- 47.Sigurdsson A, Le KT, Woo SM, Rassoulian SA, McLachlan K, Abbassi F, Garland RW. Six-month healing success rates after endodontic treatment using the novel GentleWave™ system: the pure prospective multi-center clinical study. J Clin Exp Dent. 2016;8:e290–e298. doi: 10.4317/jced.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sigurdsson A, Garland RW, Le KT, Woo SM. 12-month healing rates after endodontic therapy using the novel GentleWave system: a prospective multicenter clinical study. J Endod. 2016;42:1040–1048. doi: 10.1016/j.joen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Sigurdsson A, Garland RW, Le KT, Rassoulian SA. Healing of periapical lesions after endodontic treatment with the GentleWave procedure: a prospective multicenter clinical study. J Endod. 2018;44:510–517. doi: 10.1016/j.joen.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Martins MR, Carvalho MF, Vaz IP, Capelas JA, Martins MA, Gutknecht N. Efficacy of Er,Cr:YSGG laser with endodontical radial firing tips on the outcome of endodontic treatment: blind randomized controlled clinical trial with six-month evaluation. Lasers Med Sci. 2013;28:1049–1055. doi: 10.1007/s10103-012-1172-6. [DOI] [PubMed] [Google Scholar]
- 51.Siqueira JF, Jr, Rôças IN, Ricucci D, Hülsmann M. Causes and management of post-treatment apical periodontitis. Br Dent J. 2014;216:305–312. doi: 10.1038/sj.bdj.2014.200. [DOI] [PubMed] [Google Scholar]
- 52.Swimberghe RCD, Coenye T, De Moor RJG, Meire MA. Biofilm model systems for root canal disinfection: a literature review. Int Endod J. 2019;52:604–628. doi: 10.1111/iej.13050. [DOI] [PubMed] [Google Scholar]
- 53.Huffaker SK, Safavi K, Spangberg LS, Kaufman B. Influence of a passive sonic irrigation system on the elimination of bacteria from root canal systems: a clinical study. J Endod. 2010;36:1315–1318. doi: 10.1016/j.joen.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Rico-Romano C, Zubizarreta-Macho Á, Baquero-Artigao MR, Mena-Álvarez J. An analysis in vivo of intracanal bacterial load before and after chemo-mechanical preparation: a comparative analysis of two irrigants and two activation techniques. J Clin Exp Dent. 2016;8:e9–e13. doi: 10.4317/jced.52585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beus C, Safavi K, Stratton J, Kaufman B. Comparison of the effect of two endodontic irrigation protocols on the elimination of bacteria from root canal system: a prospective, randomized clinical trial. J Endod. 2012;38:1479–1483. doi: 10.1016/j.joen.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Cohenca N, Silva LA, Silva RA, Nelson-Filho P, Heilborn C, Watanabe E, Saraiva MC. Microbiological evaluation of different irrigation protocols on root canal disinfection in teeth with apical periodontitis: an in vivo study. Braz Dent J. 2013;24:467–473. doi: 10.1590/0103-6440201302179. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura VC, Pinheiro ET, Prado LC, Silveira AC, Carvalho APL, Mayer MPA, Gavini G. Effect of ultrasonic activation on the reduction of bacteria and endotoxins in root canals: a randomized clinical trial. Int Endod J. 2018;51(Supplement 1):e12–e22. doi: 10.1111/iej.12783. [DOI] [PubMed] [Google Scholar]
- 58.Shen Y, Stojicic S, Haapasalo M. Bacterial viability in starved and revitalized biofilms: comparison of viability staining and direct culture. J Endod. 2010;36:1820–1823. doi: 10.1016/j.joen.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 59.Carver K, Nusstein J, Reader A, Beck M. In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. J Endod. 2007;33:1038–1043. doi: 10.1016/j.joen.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 60.Paiva SS, Siqueira JF, Jr, Rôças IN, Carmo FL, Leite DC, Ferreira DC, Rachid CT, Rosado AS. Molecular microbiological evaluation of passive ultrasonic activation as a supplementary disinfecting step: a clinical study. J Endod. 2013;39:190–194. doi: 10.1016/j.joen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Paiva SS, Siqueira JF, Jr, Rôças IN, Carmo FL, Ferreira DC, Curvelo JAR, Soares RMA, Rosado AS. Supplementing the antimicrobial effects of chemomechanical debridement with either passive ultrasonic irrigation or a final rinse with chlorhexidine: a clinical study. J Endod. 2012;38:1202–1206. doi: 10.1016/j.joen.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 2007;33:782–787. doi: 10.1016/j.joen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31:166–170. doi: 10.1097/01.don.0000137651.01496.48. [DOI] [PubMed] [Google Scholar]
- 64.Pawar R, Alqaied A, Safavi K, Boyko J, Kaufman B. Influence of an apical negative pressure irrigation system on bacterial elimination during endodontic therapy: a prospective randomized clinical study. J Endod. 2012;38:1177–1181. doi: 10.1016/j.joen.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Cohenca N, Heilborn C, Johnson JD, Flores DS, Ito IY, da Silva LA. Apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing on root canal disinfection in dog teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e42–e46. doi: 10.1016/j.tripleo.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Granevik Lindström M, Wolf E, Fransson H. The antibacterial effect of Nd:YAG laser treatment of teeth with apical periodontitis: a randomized controlled trial. J Endod. 2017;43:857–863. doi: 10.1016/j.joen.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Pourhajibagher M, Ghorbanzadeh R, Parker S, Chiniforush N, Bahador A. The evaluation of cultivable microbiota profile in patients with secondary endodontic infection before and after photo-activated disinfection. Photodiagnosis Photodyn Ther. 2017;18:198–203. doi: 10.1016/j.pdpdt.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Pourhajibagher M, Raoofian R, Ghorbanzadeh R, Bahador A. An experimental study for rapid detection and quantification of endodontic microbiota following photo-activated disinfection via new multiplex real-time PCR assay. Photodiagnosis Photodyn Ther. 2018;21:344–350. doi: 10.1016/j.pdpdt.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Pourhajibagher M, Bahador A. An in vivo evaluation of microbial diversity before and after the photo-activated disinfection in primary endodontic infections: traditional phenotypic and molecular approaches. Photodiagnosis Photodyn Ther. 2018;22:19–25. doi: 10.1016/j.pdpdt.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 70.Asnaashari M, Ashraf H, Rahmati A, Amini N. A comparison between effect of photodynamic therapy by LED and calcium hydroxide therapy for root canal disinfection against Enterococcus faecalis: a randomized controlled trial. Photodiagnosis Photodyn Ther. 2017;17:226–232. doi: 10.1016/j.pdpdt.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Jurič IB, Plečko V, Pandurić DG, Anić I. The antimicrobial effectiveness of photodynamic therapy used as an addition to the conventional endodontic re-treatment: a clinical study. Photodiagnosis Photodyn Ther. 2014;11:549–555. doi: 10.1016/j.pdpdt.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Garcez AS, Nuñez SC, Hamblim MR, Suzuki H, Ribeiro MS. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: a preliminary report. J Endod. 2010;36:1463–1466. doi: 10.1016/j.joen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 73.López FU, Kopper PM, Bona AD, Steier L, de Figueiredo JA, Vier-Pelisser FV. Effect of different irrigating solutions and photo-activated therapy for in vivo root canal treatment. Braz Dent J. 2015;26:228–233. doi: 10.1590/0103-6440201300154. [DOI] [PubMed] [Google Scholar]
- 74.Decurcio DA, Rossi-Fedele G, Estrela C, Pulikkotil SJ, Nagendrababu V. Machine-assisted agitation reduces postoperative pain during root canal treatment: a systematic review and meta-analysis from randomized clinical trials. J Endod. 2019;45:387–393.e2. doi: 10.1016/j.joen.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Ramamoorthi S, Nivedhitha MS, Divyanand MJ. Comparative evaluation of postoperative pain after using endodontic needle and EndoActivator during root canal irrigation: a randomised controlled trial. Aust Endod J. 2015;41:78–87. doi: 10.1111/aej.12076. [DOI] [PubMed] [Google Scholar]
- 76.Topçuoğlu HS, Topçuoğlu G, Arslan H. The effect of different irrigation agitation techniques on postoperative pain in mandibular molar teeth with symptomatic irreversible pulpitis: a randomized clinical trial. J Endod. 2018;44:1451–1456. doi: 10.1016/j.joen.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Al-Zaka IM. The incidence of pain after root canal treatment using different irrigation methods. Tikrit J Dent Sci. 2012;1:38–43. [Google Scholar]
- 78.Yılmaz K, Tüfenkçi P, Adıgüzel M. The effects of QMix and EndoActivator on postoperative pain in mandibular molars with nonvital pulps: a randomized clinical trial. Clin Oral Investig. 2019;23:4173–4180. doi: 10.1007/s00784-019-02856-6. [DOI] [PubMed] [Google Scholar]
- 79.Middha M, Sangwan P, Tewari S, Duhan J. Effect of continuous ultrasonic irrigation on postoperative pain in mandibular molars with nonvital pulps: a randomized clinical trial. Int Endod J. 2017;50:522–530. doi: 10.1111/iej.12666. [DOI] [PubMed] [Google Scholar]
- 80.Coelho MS, Vilas-Boas L, Tawil PZ. The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps. Photodiagnosis Photodyn Ther. 2019;27:396–401. doi: 10.1016/j.pdpdt.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Gondim E, Jr, Setzer FC, Dos Carmo CB, Kim S. Postoperative pain after the application of two different irrigation devices in a prospective randomized clinical trial. J Endod. 2010;36:1295–1301. doi: 10.1016/j.joen.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Topçuoğlu HS, Topçuoğlu G, Arslan H. The effect of apical positive and negative pressure irrigation methods on post-operative pain in mandibular molar teeth with symptomatic irreversible pulpitis-a randomized clinical trial. J Endod. 2018;44:1210–1215. doi: 10.1016/j.joen.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]