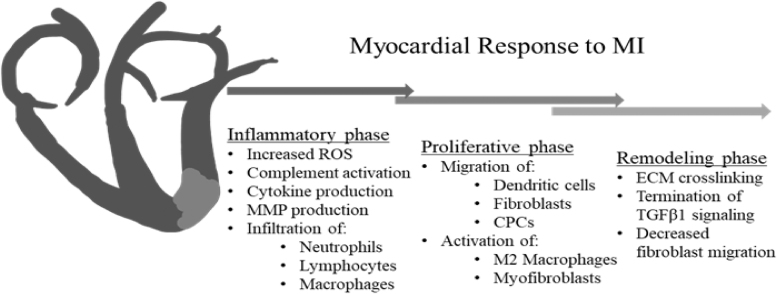
Figure 1.
Myocardial response to MI Following myocardial infarction, the heart progresses through overlapping phases of inflammation, proliferation, and remodeling, similar to the response observed in dermal wounds. Cardiomyocyte-derived inflammatory mediators lead to increased production of ROS, release of proinflammatory cytokines, and complement activation. Endothelial activation stimulates additional cytokine and ROS production, as well as infiltration of neutrophils, lymphocytes, mast cells, and macrophages into the IA. MMP production leads to ECM breakdown, with collagen and fibronectin fragments contributing to the continued inflammatory response. During the proliferative phase, the MI, proinflammatory macrophages are thought to transition to a more reparative M2 phenotype, with secretion of anti-inflammatory and profibrotic growth factors decreasing the fibrotic response. Secretion of potent chemokines, such as SDF1α, recruits the migration of CPCs to the IA. Infiltration of dendritic cells that secrete the anti-inflammatory cytokine IL-10 contributes to this M2 macrophage transition. Secretion of profibrotic growth factors such as TGFB1, as well as activation of the renin-angiotensin-aldosterone pathway lead to recruitment of fibroblasts to the infarct area and subsequent activation of fibroblasts to the highly contractile, profibrotic myofibroblast phenotype. The remodeling phase is characterized by continued ECM deposition and crosslinking, with downregulation of TGFB1 signaling, and decreased cellular infiltration as the increased ECM crosslinking inhibits cellular migration. CPC, cardiac progenitor cell; ECM, extracellular matrix; ROS, reactive oxygen species; MMP, matrix metalloproteinase; SDF1α, stromal-derived factor 1 α; TGFB-1, transforming growth factor beta-1; IL-10, interleukin-10; IA, infarct area.