Figure 1.
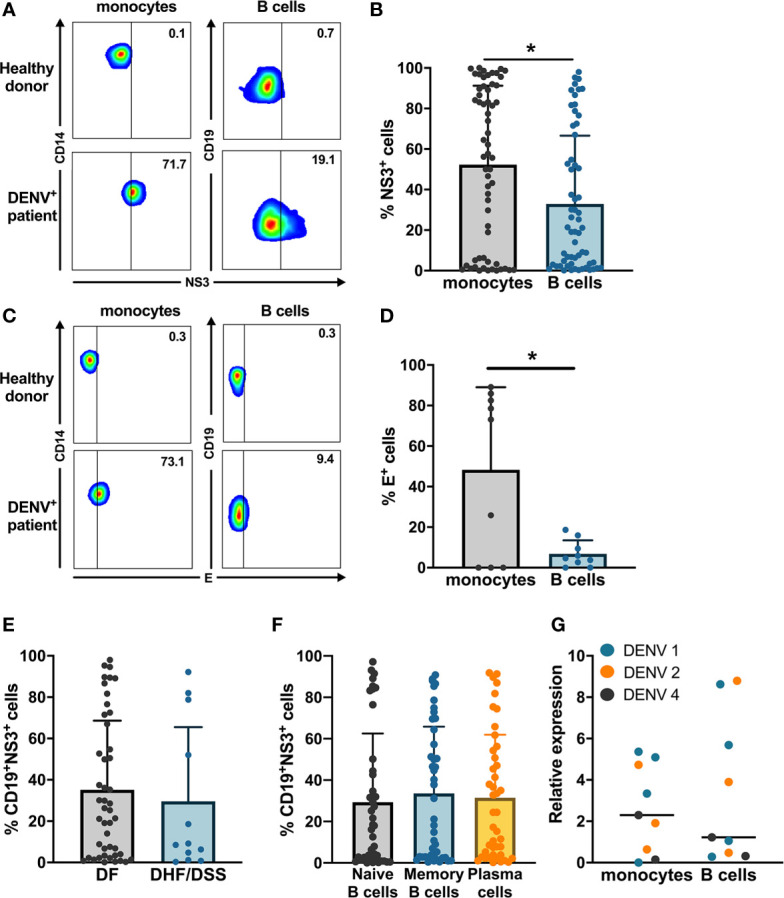
Ex vivo detection of DENV in B cells from dengue patients. PBMCs from patients in the acute phase of DENV infection (n = 60) were stained on the surface with antibodies for immune cell markers and intracellularly with anti-DENV NS3 antibody or pan flaviviral fusion loop specific 4G2 antibody. (A, B) Representative plots for NS3 staining in CD14+ monocytes and CD19+ B cells. The percentage of NS3+ cells were determined for CD14+ monocytes and CD19+ B cells. (C, D) Representative plot for anti-E staining in CD14+ monocytes and CD19+ B cells. Percentages of E+ monocytes and B cells were determined in a subset of dengue patients (n = 9). (E) DENV patients were classified as DF (n = 46) and DHF/DSS (n = 12) as per WHO 1997 classification, and the percentage of CD19+NS3+ cells was determined. p-values were calculated using Mann–Whitney U test for comparing two groups. (F) B cells from dengue patients were gated for naive B cells (CD19+CD27−), memory B cells (CD19+CD27+CD138−) and plasma cells (CD19+CD27+CD138+), and the percentage of NS3+ cells was determined. (G) CD14+ monocytes and CD19+ B cells were isolated from PBMCs from dengue patients by magnetic sorting. RT-qPCR was done for DENV by serotype-specific PCR and HPRT. Relative expression was calculated using 2−ΔΔCt method. For all panels, P-values were calculated using Mann–Whitney U test for comparing two groups. Bars and lines represent mean and standard deviation (SD). (*P < 0.05).
