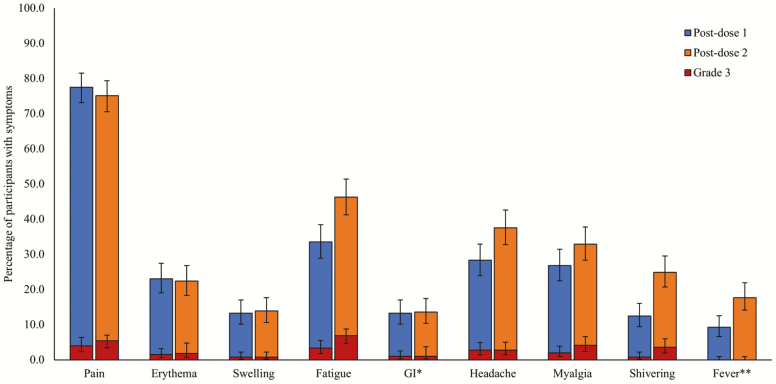
Figure 1.
Percentage of participants with solicited local and systemic AEs over the 7-day follow-up period post-dose 1 and post-dose 2 (total vaccinated cohort). AE, adverse event; *nausea, vomiting, diarrhea and/or abdominal pain; **≥37.5°C; # RZV Dose 1 administered on Day 0, Dose 2 administered on Day 60. Error bars represent 95% confidence intervals.