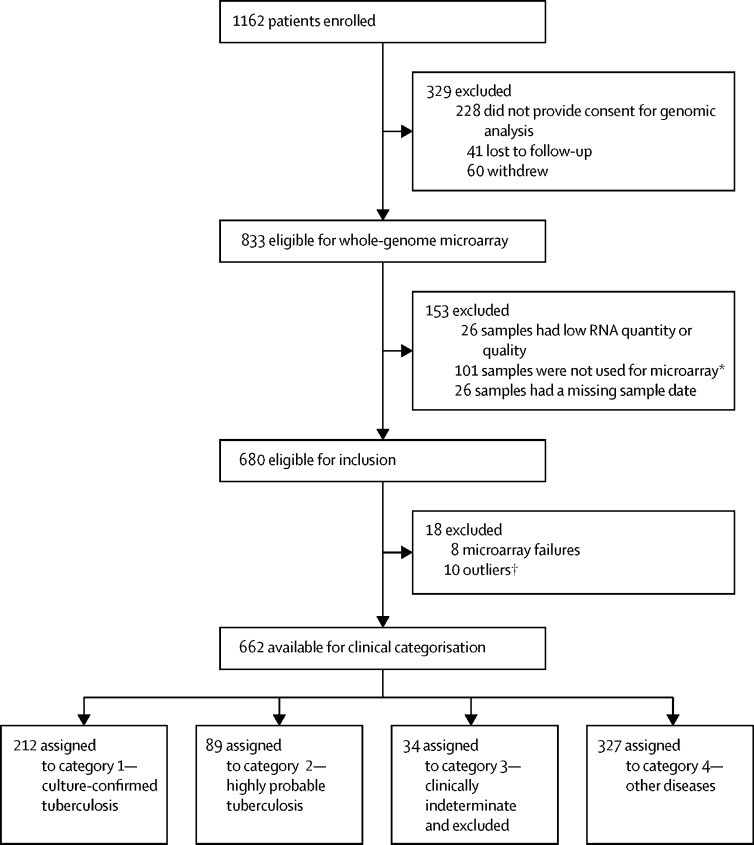
Figure 1.
Study profile
*Recruitment was extended from Aug 31, 2013 (as used and reported in Whitworth et al16), to Dec 31, 2014, to maximise HIV-positive tuberculosis cases to have sufficient numbers to compare the technologies being evaluated in HIV-positive individuals. However, by Dec 31, 2014, this strategy only delivered 20 cases of HIV-positive tuberculosis, still fewer than the number required to meaningfully compare the subgroups. Therefore, 25 HIV-positive participants with tuberculosis who were excluded were randomly selected from the total number of 126 such cases to form a balanced group relative to the 20 HIV-positive tuberculosis cases, meaning that 101 HIV-positive participants with tuberculosis were excluded at this stage. This study included eight HIV-positive patients recruited during this extension period not included in Whitworth et al,16 one of whom had tuberculosis. †Defined as falling outside the 95% confidence ellipse on principle component analysis.