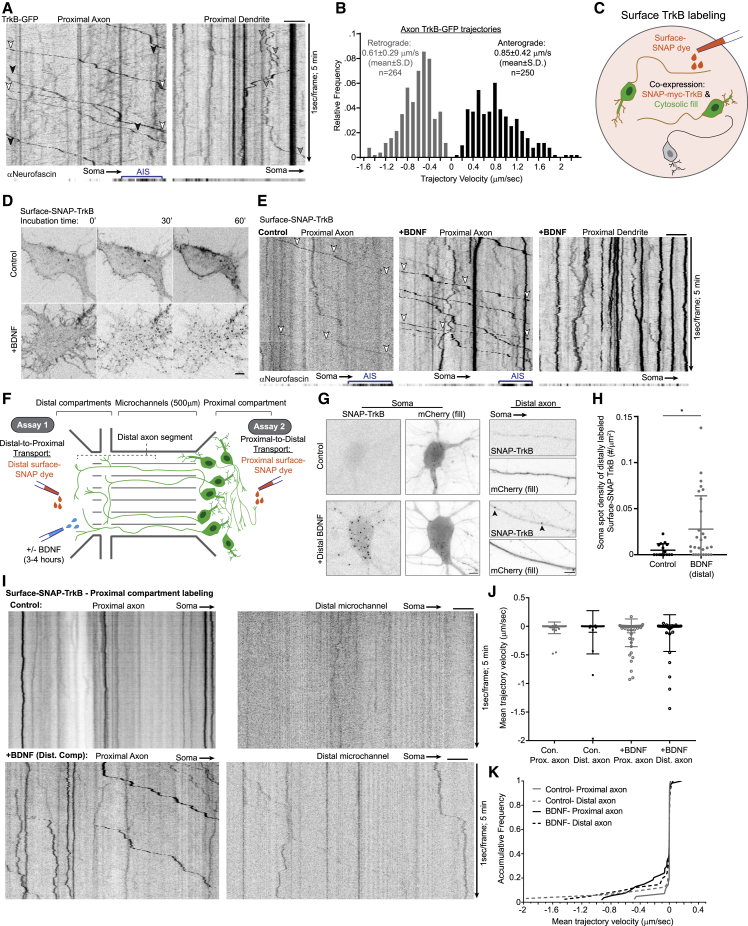
Figure 1.
Axonal transport of internalized TrkB receptors is predominantly retrograde
(A) Kymographs depict bidirectional motility of TrkB-GFP in the proximal axon and dendrite. Neurofascin signal (extracellular antibody) was acquired at the first time point and is projected to localize the AIS. TrkB-GFP signal was acquired at 1 s/frame for 5 min. Examples of processively moving retrograde and anterograde TrkB cargos in the axon are marked by white and black arrowheads, respectively. Example of bidirectional trajectory of TrkB-GFP in the dendrite is marked by gray arrowheads.
(B) Histogram of TrkB-GFP trajectory velocities undergoing processive movement based on the analysis of 514 trajectories from 11 axons collected from 2 independent cultures.
(C) Scheme of surface-labeling setup used in experiments shown in (D) and (E). Neurons expressing SNAP-TrkB were labeled with surface-SNAP dye to follow the localization and transport of TrkB from the plasma membrane.
(D) BDNF induces the internalization and accumulation of cell-surface TrkB in intracellular compartments. Time-lapse images of surface-SNAP-TrkB-labeled cells treated with bath application of control media or media supplemented with BDNF. Images were taken at t = 0, 30, and 60 min after treatment.
(E) Kymographs of SNAP-TrkB motility in the proximal axon and dendrites. Neurons were pulsed labeled with surface-SNAP then treated with BDNF or control media, then imaged at a time window of 45–90 min later. Neurofascin is imaged and presented as in (A). White arrowheads mark retrograde transport of internalized TrkB.
(F) Scheme of compartmental microfluidic chamber (MFC) setup used in experiments shown in (G) and (H) depicted under (1) and (I–K) under (2). Neurons were plated in the proximal compartment, with their axon projecting into the distal compartment. Surface-SNAP labeling was carried specifically in either the proximal or the distal axon compartment to follow axon-to-soma or soma-to-axon transport. Control or BDNF supplemented media was added in the distal compartment.
(G) BDNF induces somatic accumulation of retrogradely transported TrkB. Axons of SNAP-TrkB-expressing neurons were surface labeled in the distal axon and treated with either control or BDNF supplemented media for 2 h in the distal axon then fixed. mCherry was used to label neuron morphology. Arrowheads mark SNAP-TrkB puncta appearing in the distal axon in response to BDNF.
(H) Scatter plot of mean ± SEM of density of SNAP-TrkB spots counted in somas with axons crossing into the distal compartment after surface-SNAP labeling in the distal compartment. ∗Student’s t test, p < 0.05, n = 16 and 28 somas, from 6 and 13 independent MFC for control and BDNF groups, respectively)
(I) Kymographs of surface-labeled SNAP-TrkB mobility along proximal and distal axon segments. Neurons cultured in MFC and transfected with SNAP-TrkB were surface labeled in the proximal compartment, followed by treatment with either control or BDNF media in the distal compartment for 2–4 h before live imaging.
(J) Scatter plot of mean ± SEM trajectory velocity measured for individual SNAP-TrkB trajectories.
(K) Cumulative frequency plots of mean trajectory velocity. Data in (I–K) are based on a pool of 42, 32, 54, and 41 trajectories from 6, 7, 8, and 10 axon segments for proximal control, distal control, proximal +BDNF proximal and distal +BDNF conditions, respectively, collected from 5 and 4 independent MFC cultures for control and +BDNF conditions, respectively. All scale bars, 5 μm.