Figure 2. Clinical Tiering of Molecular Alterations Identified in Metastatic Cancer.
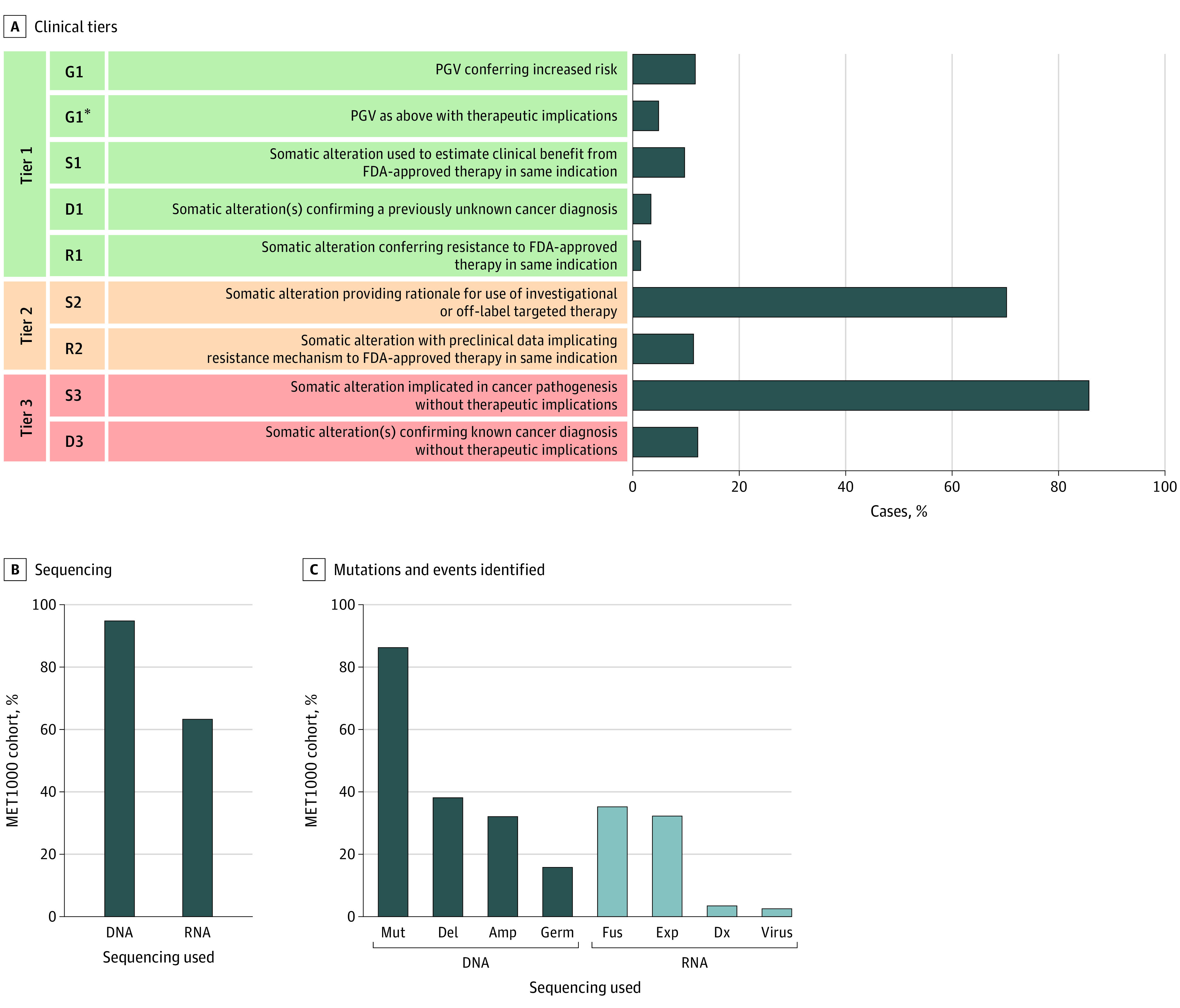
A, Tiering of genomic alterations identified in the MET1000 cohort by clinical relevance. D indicates diagnosis change; G, germline; R, resistance to therapy; and S, somatic. Tier 1 alterations were known to have clinical utility for that individual’s cancer type and included pathogenic germline variants (PGVs) conferring increased cancer risk, changes in cancer diagnosis, and somatic alteration(s) used to estimate clinical benefit from or resistance to a therapy approved by the US Food and Drug Administration (FDA). Tier 2 alterations included somatic events that provided rationale for use of investigational or off-label targeted therapy and alterations postulated from strong preclinical evidence to estimate resistance to an FDA-approved therapy in that indication. Tier 3 included alterations implicated in cancer pathogenesis or molecular events indicative of a known cancer diagnosis but without current therapeutic implications. Genomic alterations in tiers 1 and 2 were considered potentially clinically actionable. B, Percentage of cases in which DNA or RNA sequencing contributed to identifying clinically relevant alterations. C, Classes of clinically relevant alterations identified in the MET1000 cohort. Amp indicates amplification; Del, homozygous deletion; Dx, markers for cancer of unknown primary origin or change of diagnosis; Exp, expression concordant with gene amplification; Fus, gene fusion; Germ, germline; Mut, mutation; and Virus, viral pathogen.
