Figure 4. Pathogenic Germline Variants (PGVs) Observed in the MET1000 Cohort.
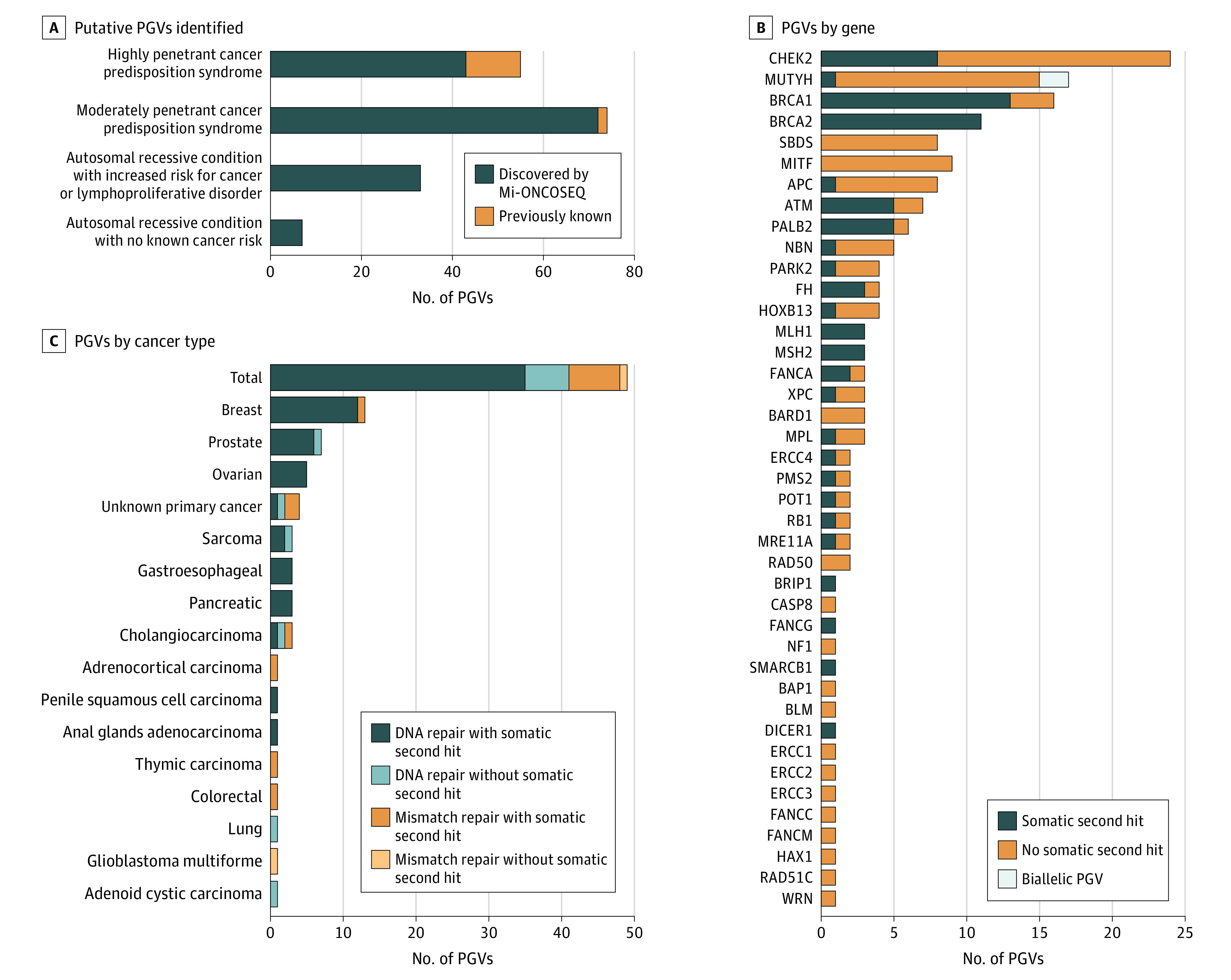
A, Among 1015 patients undergoing sequencing, 169 putative PGVs were identified (160 patients [15.8%] of MET1000 cohort). Fifty-five PGVs associated with highly penetrant cancer predisposition syndromes (including PGVs in APC, BAP1, BRCA1, BRCA2, DICER1, FH, HOXB13, MLH1, MSH2, PALB2, PMS2, POT1, and RB1) were identified, which included 14 PGVs known before the patient’s enrollment in the Michigan Oncology Sequencing Program (Mi-ONCOSEQ). Seventy-four PGVs associated with moderately penetrant cancer predisposition syndromes (including PGVs in APC, ATM, BARD1, CHEK2, MITF, MRE11A, MUTYH, RAD50, RAD51C, NF1, and SMARCB1) were identified, which included 2 PGVs known before the patient’s enrollment in Mi-ONCOSEQ. An additional 33 PGVs associated with an autosomal recessive condition conferring increased risk for cancer or lymphoproliferative disorder (including MPL, BLM, CASP8, ERCC1, ERCC2, ERCC3, ERCC4, FANCA, FANCC, FANCG, FANCM, HAX1, NBN, SBDS, and XPC) and 7 PGVs associated with an autosomal recessive condition not known to increase cancer risk (including PARK2, FH, and WRN) were identified. B, Pathogenic germline variants with somatic second-hit events identified by gene. Sixty-nine of the PGVs identified (40.8% of PGVs and 6.8% of MET1000 cohort) harbored a somatic second-hit event in the tumor. Incidental PGVs in highly penetrant cancer predisposition syndromes (ie, BRCA1) were identified in cases where the PGV was not likely related to tumor pathogenesis. C, Pathogenic germline variants with therapeutic targets were identified in 49 patients (4.8% of MET1000 cohort), often in diseases not typically associated with cancer predisposition syndromes in DNA or mismatch repair. Among the 49 PGVs identified with a therapeutic target, 42 (85.7%) harbored a somatic second-hit event in the tumor.
