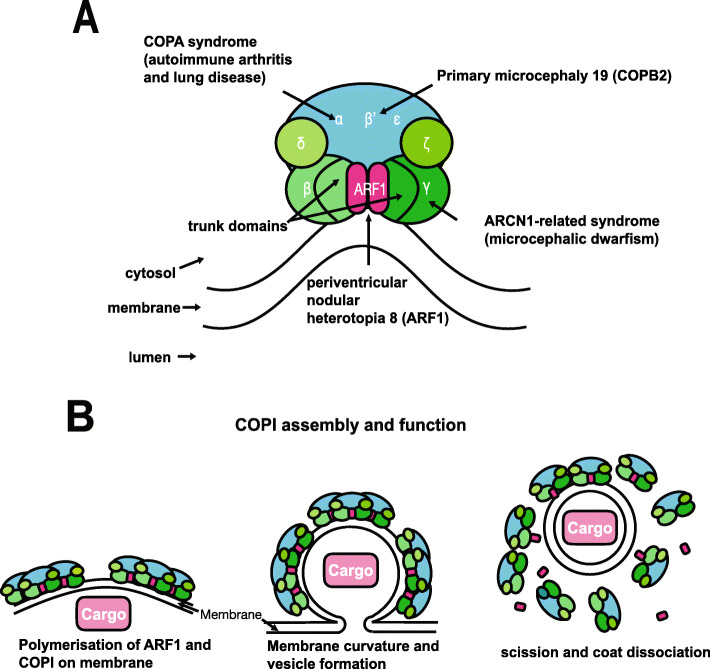
Fig. 1.
Structure and function of the COP1 complex. a COPI consists of a scaffold “B-subcomplex” (blue) and an adaptor “F-subcomplex” (green). When GTP-bound, two ARF1 small GTPase molecules associate with the membrane and bind COPI via the β-COP and γ-COP subunits. A number of subunits of this complex have been implicated in human disease as shown. b COPI complexes and their ARF1 molecules associate into triads. Cargo, such as ER-resident proteins which need to be returned from the Golgi, are selected by binding directly with COPI subunits or indirectly through transmembrane receptors which in turn bind with COPI. COPI polymerises on the membrane enabling its deformation/curvature, and eventually budding and scission of the transport vesicle. When released, the vesicle’s coat is shed and ARF1 and COPI dissociate. Adapted from Nickel et al. [12]