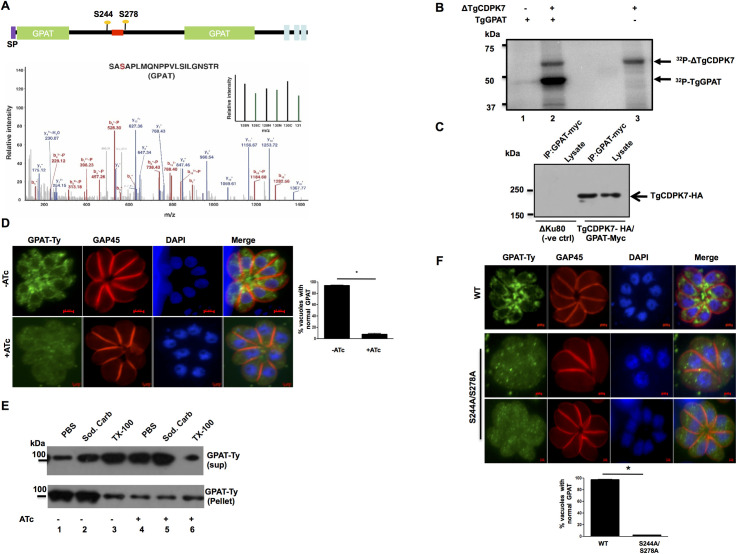
Fig 6. TgCDPK7 interacts with TgGPAT and regulates its localization.
A. Domain architecture of TgGPAT (ToxoDB ID: TGGT1_256980) indicating N-terminal signal peptide and three transmembrane domains. The domain analysis suggested that its GPAT domain is split due to the presence of an insert. Multiple sites (S244, S278) on GPAT protein were identified to be hypophosphorylated upon TgCDPK7 depletion that were located in the insert. The location of the myc/Ty tag introduced in TgGPAT between aa 256 and 257 is indicated in red. Bottom Panel: MS/MS spectra for S278, one of the sites which was hypophosphorylated upon TgCDPK7 depletion (green bars). B. A deletion mutant of TgCDPK7 (ΔTgCDPK7) that has the PH and kinase domain was used to phosphorylate recombinant fragment of GPAT as a GST tagged protein in vitro using γ32P-labelled ATP. The reaction mixture was separated by SDS-PAGE and gel was used for phosphorimaging. Autophosphorylation of the kinase was also observed. C. A myc-tag was introduced in TgGPAT gene after a.a 256 using CRISPR-Cas9 in C-terminally HA tagged TgCDPK7 parasite line. Parasite lysates were prepared from these or parental (ΔKu80) parasites and were used for IP with anti-myc antibody. Subsequently, Western blotting was performed on total lysate and GPAT-myc IP using anti-HA antibody to detect TgCDPK7-HA. A band corresponding to TgCDPK7-HA was observed only in GPAT-myc IP from transgenic parasites, which was the same size as the band in the whole cell lysates. No band was observed in the case of ΔKu80 parasites (negative control). D. GPAT was Ty-tagged at endogenous locus as described in panel A in TgCDPK7-iKD parasites (TgCDPK7-iKD/GPAT-Ty). ATc was added for 72h to deplete TgCDPK7 followed by IFA for GPAT-Ty. There was a significant change in the localization of GPAT from perinuclear ER like compartment (-ATc) to predominantly cytoplasm upon TgCDPK7 depletion (+ATc). Right Panel, Quantification of vacuoles containing normal perinuclear GPAT localization, from experiments described in the left panel. Data are mean ± SE of three independent experiments and at least 200 vacuoles were counted for each condition (*, n = 3, p<0.001, t-test). E. TgCDPK7-iKD/GPAT-Ty parasites were treated with ATc as described in panel D. Subsequently, parasite pellets were isolated and used for extracting proteins in PBS, sodium carbonate pH 11.0 or 1% Triton X-100. The supernatant (sup) or pellet fraction was electrophoresed and subjected to Western blotting using anti-Ty antibody to detect GPAT. F. C-terminal Ty-tagged full length TgGPAT or its S244A/S278A mutant were transiently overexpressed in ΔKu80 parasites. After 48h, parasites were fixed and IFA was performed using anti-Ty and anti-GAP45 antibodies. Bottom Panel, Quantification of vacuoles containing normal ER-like perinuclear TgGPAT or S244A/S278A staining, from experiments described in the right panel. Data are mean ± SE of three independent experiments and at least 200 vacuoles were counted for each condition (*, n = 3, p<0.001, t-test).