Table 2.
Analysis of distinct alkyl radical reactivity in Ni-bipyridine system.a
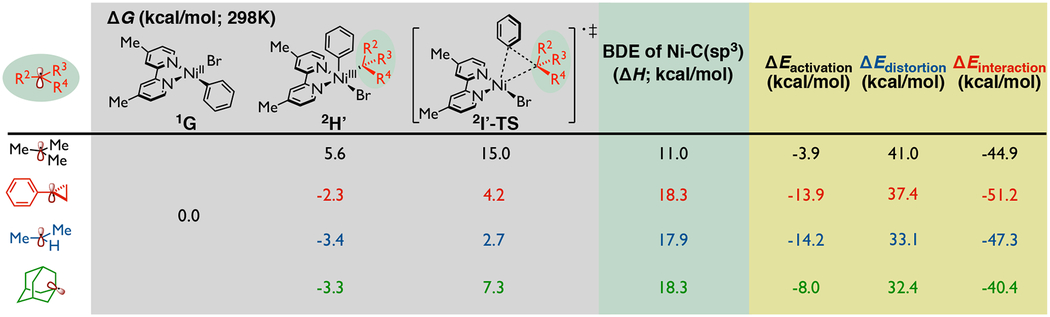
|
Gray: calculated energetics on inner-sphere reductive elimination step with different alkyl radicals; Green: related analysis on bond dissociation energy of Ni(III) intermediate; Yellow: activation strain-distortion/interaction analysis on reductive elimination transition state [relative electronic energy values were calculated with respect to the separate corresponding Ni(II) species and tert-butyl radical]. Relative free energy values were computed concerning the Ni(II) species at the UB3LYP-D3/def2-TZVPP-CPCM(THF)//UB3LYP-D3/def2-SVP-CPCM(THF) level of theory, bond dissociation energy and relative electronic energy values were calculated at the UB3LYP-D3/def2-TZVPP//UB3LYP-D3/def2-SVP-CPCM(THF) level of theory.
