Abstract
Present-day chloroplast and mitochondrial genomes contain only a few dozen genes involved in ATP synthesis, photosynthesis, and gene expression. The proteins encoded by these genes are only a small fraction of the many hundreds of proteins that act in chloroplasts and mitochondria. Hence, the vast majority, including components of organellar gene expression (OGE) machineries, are encoded by nuclear genes, translated into the cytosol and imported to these organelles. Consequently, the expression of nuclear and organellar genomes has to be very precisely coordinated. Furthermore, OGE regulation is crucial to chloroplast and mitochondria biogenesis, and hence, to plant growth and development. Notwithstanding, the molecular mechanisms governing OGE are still poorly understood. Recent results have revealed the increasing importance of nuclear-encoded modular proteins capable of binding nucleic acids and regulating OGE. Mitochondrial transcription termination factor (mTERF) proteins are a good example of this category of OGE regulators. Plant mTERFs are located in chloroplasts and/or mitochondria, and have been characterized mainly from the isolation and analyses of Arabidopsis and maize mutants. These studies have revealed their fundamental roles in different plant development aspects and responses to abiotic stress. Fourteen mTERFs have been hitherto characterized in land plants, albeit to a different extent. These numbers are limited if we consider that 31 and 35 mTERFs have been, respectively, identified in maize and Arabidopsis. Notwithstanding, remarkable progress has been made in recent years to elucidate the molecular mechanisms by which mTERFs regulate OGE. Consequently, it has been experimentally demonstrated that plant mTERFs are required for the transcription termination of chloroplast genes (mTERF6 and mTERF8), transcriptional pausing and the stabilization of chloroplast transcripts (MDA1/mTERF5), intron splicing in chloroplasts (BSM/RUG2/mTERF4 and Zm-mTERF4) and mitochondria (mTERF15 and ZmSMK3) and very recently, also in the assembly of chloroplast ribosomes and translation (mTERF9). This review aims to provide a detailed update of current knowledge about the molecular functions of plant mTERF proteins. It principally focuses on new research that has made an outstanding contribution to unravel the molecular mechanisms by which plant mTERFs regulate the expression of chloroplast and mitochondrial genomes.
Keywords: organellar gene expression, mitochondrial transcription termination factor, Arabidopsis, maize, chloroplast, mitochondria
1. Introduction
Chloroplasts and mitochondria genetic systems are the relics of the genomes of ancestral prokaryotes, which were engulfed by a primitive eukaryotic cell to establish an endosymbiotic relationship [1,2]. As the immense majority of the genes of ancestral prokaryotic genomes were transferred to the nucleus, current genomes of chloroplast (plastome) and mitochondria (mitogenome) encode only a few dozen proteins, which are principally involved in ATP production through photosynthesis and oxidative phosphorylation, respectively. Moreover, the components of gene expression machineries, such as rRNAs, tRNAs, and ribosomal proteins, are also encoded by these organellar genomes and in the case of the plastome, harbor information for a bacterial-type multi-subunit RNA polymerase (plastid encoded polymerase, PEP). Nonetheless, all these proteins represent only a tiny fraction of the hundreds of proteins present in chloroplasts and mitochondria. Hence, the vast majority of them, including the components of organellar gene expression (OGE) machineries, are encoded by nuclear genes so they are translated in cytosolic ribosomes and imported into these organelles (reviewed in [3]). Consequently, OGE apparatuses are chimeric machines that result from hundreds of millions of years of evolution since endosymbiosis events took place [4,5]. The expression and regulation of organellar genes are crucial to chloroplast and mitochondria biogenesis, and hence, to plant development and growth. In line with this, the activity of these organelles must be very fine-tuned modulated to allow plants to adjust to various physiological demands and environmental cues. Notwithstanding, the molecular mechanisms governing OGE are still poorly understood.
It is noteworthy that the majority of the nuclear-encoded protein cofactors required for OGE are unrelated to either bacterial proteins or the proteins acting in the nucleus or the cytoplasm. Furthermore, they can bind DNA or RNA in vivo, which is consistent with their function in gene expression [6,7]. Along these lines, current OGE apparatuses exhibit a mixture of gene expression features from prokaryotes, eukaryotes, and new innovations [6]. This is especially relevant in chloroplast transcriptional machinery because chloroplasts utilize a functional, multi-subunit prokaryotic-type polymerase (PEP), having core subunits encoded by the organellar genome and accessory subunits encoded by the nuclear genome, including proteins with DNA/RNA binding domains. Besides this system with remnant prokaryotic components, plastids also harbor a single subunit, nuclear-encoded, bacteriophage-type polymerase (NEP). Plant mitochondria, in contrast, use only NEPs.
The research results of recent years reveal the important role that two of these types of accessory cofactors play in OGE: pentatricopeptide repeat (PPR) proteins and mitochondrial transcription termination factors (mTERF) [8,9]. PPRs and mTERFs are eukaryotic modular proteins that belong to the same family of helical-repeat proteins that are capable of binding nucleic acids [10]. They are principally targeted to mitochondria and chloroplasts. PPRs are characterized by the presence of degenerate helical repeats of around 35 amino acids and are associated with different OGE aspects (RNA transcription, splicing, editing, and translation) that develop after the endosymbiosis event [11]. Animal genomes usually harbor 10 genes that encode PPR proteins or fewer. In contrast, the PPR family has extended in plant genomes to become one of the largest families in land plants (e.g., 650 and 450 PPRs have been identified in rice and Arabidopsis, respectively) [12].
Like PPRs, mTERFs are also modular eukaryotic proteins characterized by a variable number of tandem repetitions of a 30 amino-acid motif named “mTERF” that comprises three α-helices domains [13]. The first mTERF protein to be characterized was human MTERF1, which specifically binds to a 28-bp region immediately downstream of the mitochondrial 16S rRNA gene [14]. MTERF1 received its name because it was initially thought to promote the transcription termination of heavy strands (HS) in human mitochondrial genes and it eventually gave its name to this family of proteins. Further studies show that human MTERF1 can also be implicated in both transcription initiation and the modulation of mitochondrial DNA replication [13]. However, later works do not support a function for MTERF1 in HS transcription termination. In vivo studies in an Mterf1 knockout mouse model reveal that MTERF1 does not regulate mitochondrial rRNA synthesis at the HS. The binding of MTERF1 to the termination site would block the continuation of the light-strand promoter transcript, thereby preventing reading-through of this near full genome length transcript across its original promoter region to cause a minor effect on HS [15]. More recently, Shi et al. [16] demonstrated that MTERF1 acts as a DNA replication fork barrier to delay replication fork progression.
Additional animal MTERFs have also been characterized and grouped into four subfamilies in vertebrates, MTERF1–4, where all these proteins like MTERF1 are located in mitochondria [13,17]. Two of these subfamilies, MTERF3 and MTERF4, are also shared with invertebrates [18]. Like MTERF1, current knowledge does not support a role for MTERF2–4 in transcription termination, rather in other gene expression aspects, principally mitochondrial ribosome biogenesis in invertebrates and mammals [19,20,21]. Along these lines, MTERF2 binds to mitochondrial DNA (mtDNA) in a non-sequence-specific manner and coimmunoprecipitates with MTERF1 and MTERF3 [22,23]. MTERF2 and MTERF3, respectively, act as positive and negative regulators of mitochondrial transcription [23,24], whereas MTERF4 regulates the translation of mitochondrial genes by targeting rRNA 5-methylcytosine methyltransferase NSUN4 to the mammalian mitochondrial ribosome [19,20,25].
2. Plant mterf Defective Mutants Exhibit Developmental and/or Stress-Related Phenotypes
Unlike animals, plant mTERF functions are still poorly understood. Similarly to the aforementioned PPR proteins, land plant genomes harbor many more mTERF genes than animal genomes. In line with this, 35 mTERFs in Arabidopsis and Capsicum annuum, and 31 in maize, have been identified [26,27,28,29,30]. Plant mTERFs have been characterized to date principally from the isolation and analysis of Arabidopsis and maize mutants that show altered phenotypes. These mutant analyses have been instrumental for shedding light on the function of mTERF in plants, but also in animals (reviewed in [31]). Along these lines, plant mterf defective mutants usually exhibit stunted growth and paleness due to low chlorophyll levels when mTERFs are located in chloroplasts, and altered transcript abundance of chloroplast and/or mitochondrial genes. Furthermore, several of these mutants present developmental abnormalities. These phenotypes are likely the result of the perturbed biogenesis of chloroplasts [e.g., Arabidopsis mutants bsm/rug2/mterf4 (belaya smert/rugosa2/mterf4 [26,32]); mda1/mterf5 (mTERF defective in Arabidopsis1/mterf5 [28]); mterf6 [33,34] and mterf9/twr-2 [35]; and maize mutant Zm-mterf4 [6]] or mitochondria (e.g., Arabidopsis mutants shot1/mterf18 (suppressor of hot1-4 1/mterf18 [36]) and mterf22 [37]; and maize mutant ZmSmk3 (Zea mays small kernel 3 [38])). In line with all this, leaf morphology and internal anatomy are altered in mutants bsm/rug2/mterf4, mda1/mterf5, mterf6, and mterf9. Moreover, complete loss of mTERF6 function results in seedling lethality [33], whereas the absence of SOLDAT10 (SINGLET OXYGEN-LINKED DEATH ACTIVATOR10 [39]) or BSM/RUG2/mTERF4 [26] function leads to embryo lethality in Arabidopsis. Knockout Mterf3 or Mterf4 mice are also embryo-lethal [19,24]. Altogether, these results underpin the essential functions of mTERFs in plant and animal development. It should be noted that the numbering and classification of animal and plant mTERFs is not based on the homology between metazoans and plant mTERFs. In line with this, the classification and numbering of Arabidopsis mTERFs is due to Tatjana Kleine [27], who sorted most mTERF proteins in this species into five groups on the basis of their hypothetical functions predicted using in silico co-expression analysis and gene ontology (GO) annotations. According to this, the first two clusters were named “chloroplast” (mTERF1–9) and “chloroplast-associated” (mTERF10–12) and these mTERF are principally involved in chloroplast gene expression, embryogenesis, and protein catabolism. The “mitochondria” group members (mTERF13–19) function in DNA and RNA metabolism in this organelle, whereas those of the “mitochondrion–associated” (mTERF20–22) are related to DNA and RNA metabolism in the mitochondria and the nucleus. Finally, the members of the group dubbed “others” (mTERF23–26), are expressed at low levels and may act in the nucleus. Despite the fact that metazoan and plant mTERFs are involved in RNA or DNA metabolism in the organelles, the levels of identity and similarity between plant and metazoan mTERF proteins are rather low [28,32,35].
It is worth mentioning that altered responses to different environmental stresses have also been reported for several mterf mutants, which supports an emerging role for plant mTERFs in abiotic stress tolerance (reviewed in [12,40]). Consistently with this, mutants soldat10 and shot1/mterf8, respectively, display enhanced light sensitivity and tolerance to heat stress [36,39], whereas mutants mda1/mterf5 and mterf9/twr-2 are less sensitive to salt stress than the wild type (WT) [28,35], and mutants mterf6 and mterf10 are hypersensitive to salinity [41,42]. Numerous Arabidopsis mutants displaying enhanced or reduced tolerance to abiotic stresses also show altered sensitivity to the abscisic acid hormone (ABA). This is not unexpected given the fundamental role of ABA in plant adaptation to abiotic stress [43].
Taken together, the aforementioned mutant analyses reveal that mTERFs play a fundamental role in plant growth and development, and also in their response and adaptation to adverse environmental conditions (recently reviewed in [44]).
3. Deciphering the Molecular Functions of Plant mTERF
Linder et al. [17] described for the first time the presence of the mTERF family of proteins in photosynthetic organisms. Henceforth, 14 plant mTERFs have been characterized, albeit to a different extent, from the isolation and analysis of mutants in Arabidopsis (SOLDAT10/mTERF1 [39]; BSM/RUG2/mTERF4 [26,32]; MDA1/mTERF5 [7,28,45]; mTERF6 [33,34,41,46]; mTERF8 [47]; mTERF9 [35]; mTERF10, mTERF11 and mTERF12 [42]; mTERF15 [48]; SHOT1/mTERF18 [36] and mTERF22 [37]) and maize (Zm-mTERF4 and ZmSmk3 [6,38]). Notwithstanding, the first mTERF to be thoroughly studied in a photosynthetic organism did not belong to a land plant, but to the unicellular alga Chlamydomonas reinhardtii (mTERF-LIKE GENE OF CHLAMYDOMONAS1, MOC1 [49,50]). These numbers are still small considering that nearly 70 mTERFs have been identified in Arabidopsis and maize (see above).
Eight of the aforementioned mTERF proteins are targeted to chloroplasts (Zm-mTERF4, SOLDAT10/mTERF1, MDA1/mTERF5, mTERF8, mTERF9, mTERF10, mTERF11, mTERF12), five to mitochondria (MOC1, ZmSmk3, mTERF15, SHOT1/mTERF18, and mTERF22), and two possibly to both organelles (BSM/RUG2/mTERF4 and mTERF6). Nevertheless, a precise molecular function has been assigned to only a handful of them: BSM/RUG2/mTERF4, MDA1/mTERF5, mTERF6, mTERF8, mTERF9, mTERF15, Zm-mTERF4, and ZmSMK3 (Table 1 and Figure 1). The molecular mechanisms of action of the remaining mTERFs for which a mutant phenotype has been described remain to be discovered. The present results indicate that plant mTERFs participate in OGE regulation at transcriptional or post-transcriptional levels. In the former, the protein MDA1/mTERF5 promotes the transcription of chloroplast genes [7,45], whereas mTERF6 and mTERF8 are required for transcription termination in this organelle [33,46,47]. In the latter, BSM/RUG2/mTERF4, mTERF15, Zm-mTERF4, and ZmSmk3 participate in RNA splicing in chloroplasts or mitochondria [6,26,38,48], whereas mTERF9 promotes chloroplast ribosomal assembly and translation [51].
Table 1.
Plant mTERFs molecularly characterized.
| mTERF | Species | Localization | Molecular Function | Target 1 | Reference |
|---|---|---|---|---|---|
| BSM/RUG2/mTERF4 | Arabidopsis thaliana | C, M | Splicing of the second clpP group IIa intron | Binds non-specifically to chloroplast DNA | [26,32] |
| MDA1/mTERF5 | Arabidopsis thaliana | C | Transcriptional pausing in the psbEFLJ chloroplast polycistron | A sequence spanning from +30 to +51 in relation to the transcription start site of psbEFLJ operon for promoter 1 | [45] |
| Stabilization of the 5′-end of mature psbEFLJ and ndhA mRNAs | DNA sequences located near the psbE and ndhA promoters | [7] | |||
| mTERF6 | Arabidopsis thaliana | C | Maturation and promotion of transcription termination of chloroplast isoleucine transfer RNA (trnI.2) | trnI.2 dsDNA sequences; trnI.2 RNA | [33] |
| rpoA polycistron transcription termination factor | 3′-end sequence of the rpoA polycistron | [46] | |||
| mTERF8 | Arabidopsis thaliana | C | Transcription termination of the chloroplast psbEFLJ polycistron | Downstream of the stop codon of psbJ gene near the termination site | [47] |
| mTERF9 | Arabidopsis thaliana | C | Promotion of chloroplast ribosome assembly and translation | 16S, and to a lesser extent, 23S rRNAs | [51] |
| mTERF15 | Arabidopsis thaliana | M | Mitochondrial nad2 intron 3 splicing | Domain I of mitochondrial nad2 intron 3 transcript | [48] |
| Zm-mTERF4 | Zea mays | C | Splicing of chloroplast group II introns | Chloroplast group II introns | [6] |
| ZmSMK3 | Zea mays | M | Mitochondrial nad1 intron 4 and nad4 intron 1 splicing | Not determined | [38] |
1 Experimentally demonstrated by protein-nucleic acids binding assays. C: chloroplast; M: mitochondria.
Figure 1.
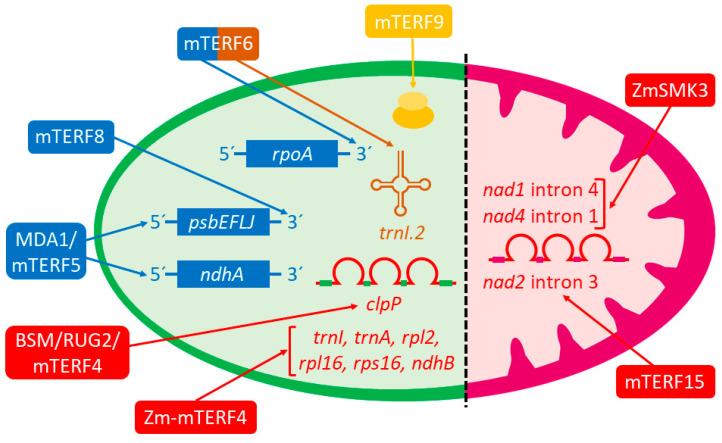
Schematic representation of the molecular functions of plant mTERFs. Colors of mTERF proteins and arrows refer to their activity as regulators of (i) transcription (blue), functioning as transcriptional pausing and stabilization (MDA1/mTERF5) or terminator (mTERF6 and mTERF8) factors of chloroplast genes (psbEFLJ polycistron, ndhA gene, and rpoA operon), (ii) group II intron splicing (red) of chloroplast (BSM/RUG2/mTERF4 and Zm-mTERF4) or mitochondria (mTERF15 and ZmSMK3) clpP, trnI, trnA, rpl2, rpl16, rps16, nad1, ndhB, nad1, nad2 and nad4 genes or (iii) translation (orange) of chloroplasts (mTERF9) promoting ribosomal assembly. mTERF6 also functions in trnI.2 maturation (brown). Green and purple semi-ovals represent, respectively, chloroplasts and mitochondria.
We report hereafter the molecular phenotypes of the mterf mutants described to date and the different experimental approaches carried out to elucidate the molecular function of mTERF proteins in photosynthetic organisms (for complementary reviews, see [44] and [52]).
4. Arabidopsis mTERF6 and mTERF8 Mediates the Transcription Termination of Chloroplast Genes
Chloroplast transcription is a complex process that involves two types of DNA-dependent RNA polymerases, as well as a number of accessory proteins encoded by the nucleus (see the Introduction; reviewed in [5]). As the transcription of plastome genes is fundamental for plant survival, it must be very carefully modulated. One of the least understood steps in regulating chloroplast RNA synthesis is transcription termination, a process that involves: (a) arrest of RNA synthesis; (b) release of the newly synthesized transcript; (c) separation of RNA polymerase from the DNA template [47]. Regulation of transcription termination is important to assure that RNA synthesis is not affected by the interference of transcripts from downstream genes and to, thus, avoid the formation of interfering antisense RNAs [53,54].
As previously mentioned, the founder member of the mTERF family was human MTERF1, to which the ability to terminate mitochondrial transcription in vitro was assigned [14]. Notwithstanding, the results obtained since then refute this idea. In fact, none of the remaining mammalian MTERFs function as termination factors of mitochondrial transcription (reviewed in [31]). In photosynthetic organisms, the detailed characterization of Chlamydomonas mTERF protein MOC1 revealed that it functions as a terminator of mitochondrial antisense transcription by binding to an octanucleotide sequence in mitochondrial rRNA coding module S3 [50].
In land plants, the first known example of a protein involved in the termination of plastid transcription was Arabidopsis RHON1, which terminates rbcL (encoding a large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase) transcription via a mechanism resembling that of the Rho factor of Escherichia coli [55]. Nonetheless, it was unknown whether, unlike animals, plant mTERFs would live up to their names by participating in transcription termination in chloroplasts and/or mitochondria. Recent elucidation of the molecular functions of Arabidopsis mTERF6 and mTERF8 has shown for the first time that mTERF proteins can terminate transcription in chloroplasts. The first piece of evidence came from the work by Romani et al. [33], who reported that recombinant mTERF6 can bind to a target dsDNA sequence located in the chloroplast isoleucine transfer (trnI.2) gene to promote in vitro transcription termination in that sequence (Table 1). Furthermore, coimmunoprecipitation experiments have revealed that mTERF6 also binds in vivo to its target sequence in the RNA of trnI.2. Interestingly, these authors discovered that trnI.2 maturation was impaired in the mterf6-1 albino mutant, which indicates that mTERF6 activity might also be fundamental for tRNA maturation (Table 1 and Figure 1).
Three years later, Zhang and colleagues demonstrated the mechanism by which mTERF6 terminates transcription in chloroplasts from the characterization of two additional mterf6 mutants. These authors reported that mTERF6 directly associates in vitro and in vivo with a 3′-end sequence of the rpoA polycistron L23-L2-S19-L22-S3-L16-L14-S8-L36-S11-rpoA, which encodes some essential ribosomal proteins and the RpoA core subunit of PEP (Table 1 and Figure 1). These authors found that the transcript levels of PEP-dependent genes were down-regulated in the mterf6-5 mutant compared to the WT, which suggests that PEP activity might be reduced in this mutant. Consistent with this hypothesis, the protein levels of RpoA were lowered in mterf6-5 individuals, which prompted Zhang et al. [46] to investigate whether mTERF6 can terminate the expression of rpoA and other genes encoding the core subunits of PEP (rpoB, rpoC1, and rpoC2), as well as some other plastid genes. To this end, they designed primer sets to characterize the non-transcribed regions of the plastome to detect potential transcription read-through in the mterf6-5 mutant. This was the case for genes rpoA, petD, ycf5, and rbcL because the transcripts from the non-transcribed spacer regions in these genes accumulated in mterf6-5 at higher levels than in the WT. Afterward, they applied chloroplast chromatin immunoprecipitation (cpChIP) to investigate whether mTERF6 can bind to the 3′-end regions of genes rpoA, petD, ycf5, and rbcL. mTERF6 enrichment was reported at the 3′ ends of rpoA, rbcL, and ycf5, suggesting that the mTERF6 protein can bind in vivo to the 3′ termini of these genes. Nonetheless, affinity was higher for rpoA. By running an electrophoretic mobility shift assay (EMSA), they confirmed that mTERF6 binding to the 3′-end region of the rpoA gene was direct. Furthermore, a conserved sequence [ATT(N)5GT] in the 3′-end of genes rpoA, rbcL, and ycf5 was identified, which suggests that mTERF6 can recognize specific sequences in its target genes. Interestingly, Romani et al. [33] had already reported that mTERF6 can specifically bind in vitro to the ATT(N)5GT sequence located within the trnI.2 gene. The low expression in the mterf6-5 mutant of rpoA polycistron and the petD gene, located downstream of the rpoA polycistron and transcribed in the opposite orientation to rpoA, prompted Zhang et al. [46] to hypothesize that mTERF6 may act as a roadblock or a bidirectional transcription-termination factor that would avoid transcription collision from two directions. Given that mTERF6 and the 3′-end regions of gene rpoA are well conserved among plant species, mTERF6 homologs may also function in transcription termination in these species [46].
mTERF8 is the second mTERF for which a role in transcription termination in chloroplasts has been demonstrated. In a paper published in 2020, Xiong and collaborators found that mTERF8, formerly pTAC15, a plastid transcriptionally active complex component [8], is localized in chloroplast nucleoids. Xiong et al. [47] showed that mTERF8 comigrates with RpoB, a core subunit of PEP, and specifically associates with the PEP complex. Analysis of the loss-of-function mutant mterf8 revealed that impaired mTERF8 causes slightly decreased photosynthetic electron flow efficiency, likely as a consequence of a reduction in the accumulation of proteins PSII PsbA and PsbB. The levels of transcripts of several plastid genes in the mterf8 mutant were altered, and the increased transcript levels of the psbJ gene were the most remarkable change compared to the WT. psbJ, as well as genes psbE, psbF, and psbL (respectively, encoding PsbJ, the α and β subunits of Cyt b559, and PsbL proteins, all of which are essential components of PSII) constitute an operon and, like psbJ, the other three genes were also up-regulated in mterf8.
When the expression of the psbEFLJ polycistron was analyzed by RNA blot employing a probe to span all the transcripts synthesized from this polycistron, a band of the same size was detected in the WT and mterf8 plants. In contrast, a larger transcript was observed only in mterf8. These results suggest the existence of read-through transcription of the psbJ gene cluster and/or perturbed RNA processing in mterf8. By a similar experimental approach to that previously employed to investigate whether mTERF6 could terminate transcription (see above), Xiong and colleagues analyzed the potential transcription read-through in mterf8. Defective transcription termination in mutant mterf8 was found as the transcription of the psbJ polycistron terminates downstream of the psbJ gene and farther than in the WT. Furthermore, circular RT-PCR and sequence analyses revealed that the termination site of the psbJ gene was located 95 nucleotides downstream of its stop codon in the WT, whereas its termination site was altered in mterf8. With EMSA and cpChIP analyses, Xiong et al. [47] discovered that mTERF8 specifically binds downstream of the stop codon of psbJ near the termination site. They also demonstrated in vitro that mTERF8 possesses transcription termination activity by acting specifically on the 3’ terminal region of the psbJ gene (Table 1 and Figure 1). As this region can form a stem-loop structure that resembles the terminators of Rho-independent transcription termination in E. coli, these authors proposed that mTERF8 could apply a similar mechanism to terminate psbJ transcription.
As mTERF6, mTERF8, and RHON1, respectively, terminate the transcription of genes rpoA, psbJ, and rbcL only, but not of other PEP-transcribed genes [46,47,55], these proteins seems to act as specific, rather than general, transcription termination factors.
5. Arabidopsis MDA1/mTERF5: A Protein That Plays a Dual Role in Transcriptional Pausing and the Stabilization of Chloroplast Transcripts
In addition to transcription initiation and termination, recent evidence shows that RNA elongation is also a widespread regulated process in bacteria and metazoan transcription [56]. Indeed, the extension of the nascent transcript is not a continuous process and the RNA polymerase usually pauses at some specific DNA sequences. This pausing delays transcript elongation and performs important regulatory functions in gene expression (e.g., facilitating the integration of cellular signals into genes by acting in signal-responsive pathways [56]). Nevertheless, it was unknown whether transcriptional pausing also occurred in chloroplasts and, if so, whether it would have a regulatory function. This scenario has recently changed due to the comprehensive work performed by Ding et al. [45], who recently elucidated the molecular mechanisms of Arabidopsis MDA1/mTERF5 action.
These authors characterized T-DNA knockout mterf5 mutants of the MDA1/mTERF5 gene, which displayed reduced growth, pale green leaves, and a defective PSII function. In line with this, mterf5 individuals showed a severe reduction in the levels of the core subunits of PSII, as well as other key photosynthetic complexes, compared to the WT. Their study of the expression of plastid genes revealed that the transcript levels of psbEFLJ polycistron (see above) were marked lower in the mterf5-1 mutant, whereas no significant differences were reported for the other studied genes. Furthermore, of all the investigated genes, only the expression patterns of the transcripts produced by the psbEFLJ promoters, as well as the transcriptional initiation rate for psbEFLJ, were perturbed in the mterf5 mutants. All this suggests that MDA1/mTERF5 specifically regulates psbEFLJ transcription (Figure 1).
By means of cpChIP-sequencing using 35S:mTERF5-HA transgenic plants and EMSA, by employing a recombinant MDA1/mTERF5 protein, it was observed that MDA1/mTERF5 binds directly to a region upstream of the psbE initiation codon. DNase I footprinting and EMSA analyses identified a 21-bp sequence as the MDA1/mTERF5 target sequence; it spanned from +30 to +51 in relation to the transcription start site (TSS) of psbEFLJ for P1 (Table 1). Interestingly, MDA1/mTERF5 specifically binds to dsDNA but not single-stranded DNA of the +30 to +51 target sequence.
To investigate whether MDA1/mTERF5 causes transcriptional pausing around the promoter region of psbEFLJ, Ding and colleagues used an in vitro transcription system, which was developed to monitor transcriptional activities from initiation to RNA extension. They concluded that MDA1/mTERF5 causes transcriptional pausing around +40 in relation to the TSS of psbEFLJ (Table 1). Furthermore, a global run-on sequencing (GRO-seq) data analysis confirmed that transcriptional pausing occurs at the promoter region of psbEFLJ in vivo, and this transcriptional pausing is dependent on the binding of MDA1/mTERF5 to its target sequence. Remarkably, the re-analysis of the public GRO-seq data revealed that transcriptional pausing occurs at nearly 30% of Arabidopsis plastid genes, which indicates that this is a general feature of plastome genes.
Using cpChIP with antibodies to recognize RpoB, the β subunit of PEP, they also detected, in the WT, a high occupancy of the PEP complex near the psbEFLJ promoter, but not in other regions of this polycistron or in mterf5-1. The use of different state-of-art molecular methods has revealed that (a) pTAC6 is an interaction partner of MDA1/mTERF5; (b) most pTAC6 is associated with the PEP complex, but also exists outside this complex; (c) pTAC6 is not required for MDA1/mTERF5 binding to its target sequence in psbEFLJ and hence, (d) pTAC6 would not be involved in transcriptional pausing. Nonetheless, MDA1/mTERF5 recruits pTAC6, but not the PEP complex, to the transcriptional pause region of psbEFLJ. Interestingly, Ding and colleagues discovered that after transcription initiation, the amount of pTAC6 associated with RpoB in the PEP complex increased, whereas the pTAC6 level associated with MDA1/mTERF5 lowered. This suggests that pTAC6 recruited by MDA1/mTERF5 is assembled in the PEP complex. These authors proposed that the additional recruiting of pTAC6 into the PEP complex would enhance PEP activity by facilitating transcription elongation and accelerating transcription. Hence, MDA1/mTERF5 would function as a positive regulator of psbEFLJ transcription.
Bacteria transcriptional regulators or pausing factors bind to non-template DNA or hairpin RNA. However, MDA1/mTERF5 binds to the dsDNA target sequence, which suggests that MDA1/mTERF5 transcriptional pausing likely represents a different regulatory mechanism [45]. Therefore, this may be a chloroplast invention that developed through evolution for accurate and specific psbEFLJ expression regulation, which is fundamental for PSII functioning.
Shortly after the publication of the aforementioned work, Méteignier et al. [7] reported novel aspects about the function of MDA1/mTERF5 by means of a reverse genetics approach combined with several molecular and biochemical techniques. They characterized an Arabidopsis T-DNA knockout line, dubbed mda1-2 (also named mterf5-1 by Ding et al. [45]; see above), which was previously described by Robles et al. [28] (see Section 2), and a second mutant allele of MDA1/mTERF5, hcf111-1 [57]. mda1-2 plants showed a moderate loss of the PsaD subunit of PSI and a substantial reduction in the subunits of complexes PSII and NDH compared to the WT. Consistent with this, photosynthetic capacities of mutants mda1 and hcf111-1 decreased. Moreover, the expression analysis of chloroplast genes in the mda1-2 and hcf111-1 plants revealed that the genes of the psbEFLJ polycistron, as well as genes ndhA and ndhI from the ndhH cluster, were specifically affected (they were all down-regulated). Like Ding et al. [45], Méteignier and collaborators studied the pattern and abundance of the transcripts of psbEFLJ by RNA blotting, and also that of the ndhH gene cluster. They mapped their 5´and 3´ends by cRT-PCR. As regards the psbEFLJ polycistron, in mda1-2 plants, these authors observed decreases in the levels of two transcripts of 1.1 and 1.4 kb compared to the WT, which was more marked for the former. Furthermore, the 1.1 kb transcript was found to be a 5′-end processed psbEFLJ mRNA, and its mapping termini revealed that the frequency of the principal psbE mRNA termini had diminished in mda1-2 compared to the WT. This was more pronounced for the 5′- than for the 3′-end. Afterward, a reduction in the abundance of the different mRNAs containing genes ndhA and ndhI was also observed in mda1-2. Interestingly, the less abundant transcripts in mda1-2 started with an ndhA 5′-end and its mapping termini showed that the frequency of the predominant ndhA 5′-end is reduced in mda1-2. Therefore, the authors concluded that MDA1/mTERF5 is required principally for the stability of the 5′-end of mature psbEFLJ and ndhA mRNAs by promoting the in vivo accumulation of the transcripts containing processed psbE and ndhA 5′-ends (Table 1). To determine if changes in transcription could contribute to the lower steady-state levels of transcripts psbE and ndhA, Méteignier and collaborators quantified psbE and ndhA transcription activity and transcript abundance in mda1-2 and the WT. Compared to the WT, they observed in mda1 that the psbE and ndhA transcription rates had lowered, although the reduction in the mutant of the steady-state levels of the psbE and ndhA transcripts was much more marked. Therefore, these results indicate that MDA1/mTERF5 promotes psbE and ndhA transcription, but principally contributes to the posttranscriptional stabilization of their 5′-end processed mRNAs in vivo (Table 1 and Figure 1).
Coimmunoprecipitation experiments and the identification of proteins by LC-MS/MS revealed that MDA1/mTERF5 is found in high molecular weight complexes and associates with TAC components, including the core subunits of PEP. Méteignier and colleagues investigated the capacity of MDA1/mTERF5 to bind nucleic acids in vitro and in vivo by gel mobility shift assays, RNA immunoprecipitation sequencing (RIP-seq), and DNA immunoprecipitation (DIP)-qPCR experiments on solubilized chloroplasts, and they drew several conclusions. First, the MDA1/mTERF5 protein is able to bind dsDNA, and also ssRNA to a lesser extent. Second, MDA1/mTERF5 is not the RNA binding protein that stabilizes the termini of mature psbEFLJ and ndhA processed mRNAs, but promotes the in vivo binding of an RNA binding protein, likely a PPR protein, to the 5′-end of processed psbE and ndhA mRNAs. Third, MDA1/mTERF5 binds specifically to DNA regions in genes psbE and ndhA. Furthermore, they precisely mapped the binding site of MDA1/mTERF5 in genes psbE and ndhA. They found 27-nt DNA sequences located near the promoters of both genes. These target sequences were located −96/−70 from psbE ATG (overlapping the transcriptional pausing site identified by Ding et al.) and +110/+136 within the ndhA ORF, and the affinity for the ndhA binding site was weaker than for psbE.
By considering the aforementioned results and those by Ding et al., Méteignier and colleagues proposed a model for the MDA1/mTERF5 function in the control of psbE expression. According to this, MDA1/mTERF5 binding to DNA near the promoter region of psbE, and downstream of a potential PPR binding site, would prevent the formation of RNA secondary structures that are deleterious for the potential PPR binding to the 5′ end of psbE mRNA. This would post-transcriptionally stabilize the processed psbE mRNAs. ndhA expression might be regulated by a similar mechanism. Therefore, MDA1/mTERF5 performs a dual function in gene expression regulation: it stimulates psbE and ndhA transcription, and also stabilizes their post-transcriptionally processed mRNAs.
6. mTERF4 Functions in Group II Intron Splicing in Arabidopsis and Maize Chloroplast Genes
Some tRNA and protein encoding genes of chloroplasts and plant mitochondria harbor group I and II introns; the latter, in turn, are divided into a and b subclasses depending on their primary and secondary structures, and their different splicing mechanisms. These introns are processed and spliced by the combined action of a set of nucleus-encoded RNA-binding proteins, including, among others, maturases, CRM (chloroplast RNA splicing and ribosome maturation) domain-containing proteins, and PPRs [58]. Several mTERFs have also been recently found to participate in organellar intron splicing.
Arabidopsis mTERF4 was the first plant mTERF reported to function in plastid splicing. Babiychuk and colleagues [26] analyzed the subcellular localization of the 35 members of the mTERF family in Arabidopsis using GFP fusions to find that 11 were chloroplast-targeted and that T-DNA insertions in four of these caused embryo lethality. The insertional lethal alleles of one of these genes, mTERF4, led to arrested embryo development and produced immature white seeds, which was why the mutant was named belaya smert (bsm, white dead in Russian). Viable mutant alleles of mTERF4, named rugosa2, have also been identified and characterized, their most conspicuous phenotypic trait being the presence of green and yellowish leaf sectors in their vegetative leaves, probably as a result of perturbed chloroplast biogenesis [32]. bsm stable shoot cultures, which grow very slowly, can be maintained in vitro by the exogenous supplementation of phytohormones in order to perform further studies. In bsm albino shoots, the transcripts of the PEP-dependent rrn16S, rrn23S, rbcL, and atpA chloroplast genes were undetectable. In contrast, the transcript levels of the clpP gene increased in bsm cells compared to the WT, while the second clpP group IIa intron was not spliced in the mutant. Like clpP, the group IIa introns present in the atpF, rpl2, and rps2 genes were not spliced in bsm. The authors proposed a direct role of BSM in the splicing of the second clpP group IIa intron (Table 1 and Figure 1), which is thought to be plastid MatK maturase-independent because the inhibition of plastid translation with spectinomycin abolishes the splicing of atpF, rpl2, and rps12 group IIa introns, but not that of the clpP gene second intron.
Shortly after the work of Babiychuk and colleagues, a role for mTERF4 in organellar splicing was reported in a second plant species. Hammani and Barkan [6] worked with maize and found two insertional non-photosynthetic mutants of the Zm-mTERF4 gene, the ortholog of the BSM/RUG2/mTERF4 Arabidopsis gene. Immunoblotting assays revealed that Zm-mTERF4, like BSM, is chloroplast-localized, primarily in the stromal fraction. Northern blotting demonstrated that plastid 16S and 23S rRNAs levels lowered in the Zm-mterf4 mutants compared to the WT, which probably causes strong plastid ribosome deficiencies, as widely reported in other albino maize mutants [59,60,61,62]. Using immunoprecipitation with a Zm-mTERF4 antibody against several stromal extracts, followed by RIP-chip analyses, Hammani and Barkan identified RNA group II introns sequences from the transcripts belonging to 16 different plastid genes as ligands of Zm-mTERF4. In order to know if splicing was compromised in these transcripts, the authors used RNA blotting hybridizations with exon probes and qRT-PCR to compare the ratio of the spliced and unspliced introns in the Zm-mterf4 mutants with those of the WT, pale green hcf7 [63], and albino iojap [64] maize mutants. These mutants were included because they displayed defective plastid ribosomes, which brought about pleiotropic effects on the splicing of plastid introns [65]. Altogether, these results led the authors to classify introns into three categories: (i) trnI, trnA, rpl2, rpl16, rps16, and ndhB introns that coimmunoprecipitate with Zm-mTERF4, whose splicing is strongly increased by Zm-mTERF4; (ii) ndhA, rps12, rpl16, rps16, and ndhB introns that are weakly enriched in coimmunoprecipitation with Zm-mTERF4, whose splicing requires no Zm-mTERF4 activity; (iii) trnK, trnG, trnV, ycf3-1, petB, and petD introns, highly enriched in Zm-mTERF4 coimmunoprecipitation, but their splicing defects are slight or difficult to evaluate due to secondary effects caused by loss of plastid ribosomes.
By using sucrose gradient-fractionated chloroplast stroma, Hammani and Barkan found Zm-mTERF4 in particles within the same size range as large intron-containing complexes, where group II intron splicing factors have been identified. To confirm that Zm-mTERF4 is found in these particles containing splicing factors, Zm-mTERF4 was immunoprecipitated from chloroplast stroma, and both pellet and supernatant fractions were analyzed by immunoblotting with antibodies against several chloroplast splicing factors. The chloroplast RNA binding proteins that shared intron ligands with Zm-mTERF4 (CAF2, CFM2, CFM3, CRS1, WHY1, RNC1, THA8, and WTF1) coimmunoprecipitated with Zm-mTERF4. However, other proteins like PPR10 and CRP1, which bind non-intronic regions on plastid transcripts, were not detected in immunoblots.
Taken together, the results by Hammani and Barkan convincingly demonstrate that Zm-mTERF4 participates in group II intron splicing in chloroplasts (Table 1 and Figure 1), and show that mTERF4 function has been conserved in dicots (Arabidopsis) and monocot (maize) plants.
7. Arabidopsis mTERF15 and Maize ZmSmk3 Are Required for Intron Splicing in Mitochondria
Nine mitochondrial genes of Arabidopsis require intron splicing for the complete maturation of their transcripts, which comprises 23 intron-splicing events. Genes rps3, cox2, ccmFc, and rpl2 contain a single intron, while genes nad1, nad2, nad4, nad5, and nad7, which are required for normal mitochondrial complex I function, contain multiple introns [66].
Arabidopsis mutant mterf15, defective in the plant-specific and mitochondrial-localized mTERF15 protein, displays stunted growth and development, small organs, delayed flowering, and sterility. These phenotypic traits may result from defective mitochondrial development and/or activity because the mTERF15 function is required for normal mitochondria biogenesis and membrane integrity [48]. To gain insight into the molecular function of mTERF15, Hsu and colleagues performed in vitro binding studies with dsDNA-cellulose and Northwestern blot analyses to investigate the dsDNA- and RNA-binding abilities of the mTERF15 protein. They found that mTERF15 functions as an RNA-, but not as a DNA-, binding protein. Using a set of primers designed by de Longevialle et al. [67], they examined by qRT-PCR the 23 mitochondrial splicing events in the mterf15 mutant, WT, and transgenic plants that complement the mutant phenotype. The only altered RNA splicing event in the mterf15 mutant was that of intron 3 of nad2, which was significantly reduced, as later confirmed by Northern blot. These authors also detected the accumulation of un-spliced nad2b transcripts in mterf15 plants, which is consistent with defective intron 3 splicing because mature nad2 mRNA contains five exons from two different transcripts, nad2a (exons 1 to 2 and intron 1) and nad2b (exons 3 to 5 and introns 3 and 4), as formed through one trans-splicing and three cis-splicing events. The interaction of mTERF15 with nad2 intron 3 was confirmed by Northwestern blotting and its RNA-binding capacity by RNA-EMSA, specifically with a fragment spanning part of the sequence of the domain I intron (Table 1 and Figure 1). As nad2 encodes a subunit of mitochondrial complex I of the electron transport chain, Hu and colleagues investigated complex I formation and activity in the mterf15 mutant. They found that a nad2 intron 3 splicing defect disrupts complex I formation and decreases its activity.
More recently, a recessive mutant of maize has been characterized, ZmSmk3, which harbors a Mutator transposon inserted in the promoter of the orthologous gene of Arabidopsis mTERF15 [38]. ZmSMK3 is required for kernel development (embryo and endosperm development are arrested in the Zmsmk3 mutant) and seedling growth because those germinated seedlings grow very slowly. With a GFP construct, Pan and colleagues found that ZmSMK3 is targeted to mitochondria. Their investigation by RT-PCR of the potential changes in the levels of 35 mitochondrial gene transcripts in the mutant and the WT revealed a drastic reduction in the levels of the nad1 and nad4 mature transcripts in Zmsmk3. On the contrary, the RNA precursors of the nad1 and nad4 mature transcripts had substantially increased. By qRT-PCR, the authors evaluated the splicing efficiencies of 22 mitochondrial introns to find that it was drastically reduced only in nad1 intron 4 and nad4 intron 1 in Zmsmk3 compared to the WT (Table 1 and Figure 1). NAD1 and NAD4 are core components of mitochondrial complex I and, similar to the Arabidopsis mterf15 mutant (see above), Zmsmk3 plants also exhibit deficient mitochondrial complex I assembly and activity, as well as impaired mitochondrial function.
Pan and colleagues propose that ZmSMK3 may function in the recognition of precursor nad4 and nad1 mRNA, and in the maintenance of the nad4 and nad1 conformation for intron splicing with the cooperation of other factors. Furthermore, these authors noticed that despite the functional conservation of mTERF15 and ZmSMK3, and the high similarity of their target introns (nad1 intron4, nad2 intron3, and nad4 intron1) in Arabidopsis and maize, these two proteins acted on different introns in these species. This finding could be attributed to differences in the structure of proteins because mTERF15 has five mTERF motifs, whereas ZmSMK3 only has two. Hence, divergence in target intron splicing would result from the variation in their protein structure.
8. Arabidopsis mTERF9 Protein Promotes Chloroplast Ribosomal Assembly and Translation
The Arabidopsis mterf9 mutants show defective vegetative development and altered responses to salt [35] and photo-oxidative stresses [68]. The mTERF9 protein, also known as TWIRT1 [69], is localized to chloroplasts [26,68]. In a very recent work, published this year, Méteignier and collaborators, who already contributed to elucidating the molecular mechanisms of MDA1/mTERF5 function [7], have performed a comprehensive analysis of the molecular function mTERF9 [51]. In this work, these authors find that the loss of function of mTERF9 causes a pleiotropic photosynthetic deficiency by comparing the PS I and PSII activity of the Arabidopsis mterf9 mutant and the WT. The use of an mTERF9-GFP fusion construct confirmed that the mTERF9 protein is targeted to chloroplasts and its emission specifically co-localized with the RAP-RFP nucleoid marker. Immunoblotting assays show that levels of most of the chloroplast protein complexes tested are reduced more than 50% in the mterf9 mutant compared to WT and complemented mterf9 plants (CP, with a WT copy of the mTERF9 gene). Furthermore, the de novo synthesis of the chloroplast proteins RbcL and D1 is also decreased in this mutant, indicating that accumulation and translation of chloroplast proteins are impaired in mterf9. Using qRT-PCR, the authors find that the steady-state levels of chloroplast gene transcripts or their splicing are not affected in mterf9 plants, with the exception of 16S and 23S rRNAs, constituents of the small and large plastid ribosomal subunits, respectively, whose levels were significantly reduced compared with the WT. To investigate a possible defect in ribosome assembly, as suggested by defective accumulation of rRNAs, Méteignier et al. performed sucrose gradient fractionation of stroma from mterf9, WT, and CP plants and polysome analysis from sucrose gradients. The results show that mTERF9 associates with chloroplast ribosomes and, in turn, promotes mRNA association with them, indicating that this protein functions in translation. Furthermore, RIP assays reveal that mTERF9 binds the 16S rRNA in vivo and to a lesser extent, the 23S rRNA. Next, the authors identified, through coimmunoprecipitation of WT and CP stromal extracts treated or not with RNase, the mTERF9 protein interactome, made up of more than 150 proteins and highly enriched with proteins involved in chloroplast ribosome biogenesis. Unexpectedly, chaperonins from the CPN60 family are some of the mTERF9 in vivo protein interactants, and Méteignier and collaborators demonstrated, by using a yeast two-hybrid assay, that mTERF9 and CPN60 chaperonins physically interact. Altogether, these results prompted these authors to propose that mTERF9 would recruit the CPN60 chaperonin complex to chloroplast ribosomes during translation to help folding of nascent proteins. Nonetheless, they do exclude that, alternatively, mTERF9 might be itself a substrate of the CPN60 complex.
This recent work extends the known repertoire of plant mTERF functions to translation, beyond their roles in the control of OGE at the RNA level, and similar to metazoan mTERFs, since MTERF3 and MTER4 are involved in mitochondrial ribosomal assembly and therefore, translation [19,20,21,25].
9. Conclusions
The gene functions that persist in the chloroplast and mitochondria genomes are essential for the activity of these organelles and, consequently, for plant life. Therefore, OGE regulation must be carried out very precisely. Given the marked genomic erosion suffered by the genomes of the endosymbionts from which chloroplasts and mitochondria originate, OGE control is principally performed by proteins encoded by nuclear genes. Of these, nucleic-acid binding proteins with a modular structure, such as PPRs and mTERFs, play a fundamental role. The molecular functions of PPR in plant OGE control, especially in RNA editing, have been extensively studied for quite some time [70,71]. However, those of plant mTERFs have only recently started to emerge. We have reviewed herein the works that mainly contributed to shed light on plant mTERF molecular functions. Their results show that mTERFs operate at different levels in regulating OGE: (i) stimulating transcription and stabilizing post-transcriptionally processed mRNA (MDA1/mTERF5); (ii) terminating transcription (mTERF6 and mTERF8); (iii) promoting tRNA maturation (mTERF6); (iv) intron splicing (BSM/RUG2/mTERF4, Zm-mTERF4, mTERF15, and ZmSMK3; or (v) chloroplast ribosomal assembly and translation (mTERF9). As mTERFs hitherto molecularly characterized in detail are only a small fraction of those identified in model plants, such as Arabidopsis and maize, in forthcoming years, new works are expected to expand our knowledge about the biochemical and molecular functions in which plant mTERF participate.
Abbreviations
OGE—organellar gene expression; PPR—pentatricopeptide repeat proteins; mTERF—mitochondrial transcription termination factor; SOLDAT10—SINGLET OXYGEN-LINKED DEATH ACTIVATOR10; BSM/RUG2—BELAYA SMERT/RUGOSA2; MDA1—mTERF DEFECTIVE IN ARABIDOPSIS 1; SHOT1—SUPPRESSOR OF HOT1-4 1; MOC1—mTERF-LIKE GENE OF CHLAMYDOMONAS1.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gray M.W. Evolution of organellar genomes. Curr. Opin. Genet. Dev. 1999;9:678–687. doi: 10.1016/S0959-437X(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 2.Race H.L., Hermann R.G., Martin W. Why have organelles retained genomes? Trends Genet. 1999;15:364–370. doi: 10.1016/S0168-9525(99)01766-7. [DOI] [PubMed] [Google Scholar]
- 3.Barkan A. Studying the structure and processing of chloroplast transcripts. Methods Mol. Biol. 2011;774:183–197. doi: 10.1007/978-1-61779-234-2_12. [DOI] [PubMed] [Google Scholar]
- 4.Liere K., Weihe A., Börner T. The transcription machineries of plant mitochondria and chloroplasts: Composition, function, and regulation. J. Plant Physiol. 2011;168:1345–1360. doi: 10.1016/j.jplph.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Börner T., Aleynikova A.Y., Zubo Y.O., Kusnetsov V.V. Chloroplast RNA polymerases: Role in chloroplast biogenesis. Biochim. Biophys. Acta. 2015;1847:761–769. doi: 10.1016/j.bbabio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Hammani K., Barkan A. An mTERF domain protein functions in group II intron splicing in maize chloroplasts. Nucleic Acids Res. 2014;42:5033–5042. doi: 10.1093/nar/gku112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Méteignier L.V., Ghandour R., Meierhoff K., Zimmerman A., Chicher J., Baumberger N., Alioua A., Meurer J., Zoschke R., Hammani K. The Arabidopsis mTERF-repeat MDA1 Protein Plays a Dual Function in Transcription and Stabilization of Specific Chloroplast Transcripts Within the psbE and ndhH Operons. New Phytol. 2020;227:1376–1391. doi: 10.1111/nph.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfalz J., Liere K., Kandlbinder A., Dietz K.J., Oelmüller R. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell. 2006;18:176–197. doi: 10.1105/tpc.105.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Q.B., Huang C., Yang Z.N. Nuclear-encoded factors associated with the chloroplast transcription machinery of higher plants. Front. Plant Sci. 2014;5:316. doi: 10.3389/fpls.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipovska A., Rackham O. Modular recognition of nucleic acids by PUF, TALE and PPR proteins. Mol. Biosyst. 2012;8:699–708. doi: 10.1039/c2mb05392f. [DOI] [PubMed] [Google Scholar]
- 11.Giegé P. Pentatricopeptide repeat proteins: A set of modular RNA-specific binders massively used for organelle gene expression. RNA Biol. 2013;10:1417–1418. doi: 10.4161/rna.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robles P., Quesada V. Transcriptional and Post-transcriptional Regulation of Organellar Gene Expression (OGE) and Its Roles in Plant Salt Tolerance. Int. J. Mol. Sci. 2019;20:1056. doi: 10.3390/ijms20051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberti M., Polosa P.L., Bruni F., Manzari C., Deceglie S., Gadaleta M.N., Cantatore P. The MTERF family proteins: Mitochondrial transcription regulators and beyond. Biochim. Biophys. Acta. 2009;1787:303–311. doi: 10.1016/j.bbabio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: Identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989;58:391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- 15.Terzioglu M., Ruzzenente B., Harmel J., Mourier A., Jemt E., López M.D., Kukat C., Stewart J.B., Wibom R., Meharg C., et al. MTERF1 binds mtDNA to prevent transcriptional interference at the light-strand promoter but is dispensable for rRNA gene transcription regulation. Cell. Metab. 2013;17:618–626. doi: 10.1016/j.cmet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Posse V., Zhu X., Hyvärinen A.K., Jacobs H.T., Falkenberg M., Gustafsson C.M. Mitochondrial transcription termination factor 1 directs polar replication fork pausing. Nucleic Acids Res. 2016;44:5732–5742. doi: 10.1093/nar/gkw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linder T., Park C.B., Asin-Cayuela J., Pellegrini M., Larsson N.G., Falkenberg M., Samuelsson T., Gustafsson C.M. A family of putative transcription termination factors shared amongst metazoans and plants. Curr. Genet. 2005;48:265–269. doi: 10.1007/s00294-005-0022-5. [DOI] [PubMed] [Google Scholar]
- 18.Peralta S., Wang X., Moraes C.T. Mitochondrial transcription: Lessons from mouse models. Biochim. Biophys. Acta. 2012;1819:961–969. doi: 10.1016/j.bbagrm.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cámara Y., Asin-Cayuela J., Park C.B., Metodiev M.D., Shi Y., Ruzzenente B., Kukat C., Habermann B., Wibom R., Hultenby K., et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 2011;13:527–539. doi: 10.1016/j.cmet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Yakubovskaya E., Guja K.E., Mejia E., Castano S., Hambardjieva E., Choi W.S., García-Díaz M. Structure of the essential MTERF4:NSUN4 protein complex reveals how an MTERF protein collaborates to facilitate rRNA modification. Structure. 2012;20:1940–1947. doi: 10.1016/j.str.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wredenberg A., Lagouge M., Bratic A., Metodiev M.D., Spåhr H., Mourier A., Freyer C., Ruzzenente B., Tain L., Grönke S., et al. MTERF3 regulates mitochondrial ribosome biogenesis in invertebrates and mammals. PLoS Genet. 2013;9:e1003178. doi: 10.1371/journal.pgen.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrini M., Asin-Cayuela J., Erdjument-Bromage H., Tempst P., Larsson N.G., Gustafsson C.M. MTERF2 is a nucleoid component in mammalian mitochondria. Biochim. Biophys. Acta. 2009;1787:296–302. doi: 10.1016/j.bbabio.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Wenz T., Luca C., Torraco A., Moraes C.T. mTERF2 regulates oxidative phosphorylation by modulating mtDNA transcription. Cell Metab. 2009;9:499–511. doi: 10.1016/j.cmet.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Park C.B., Asin-Cayuela J., Cámara Y., Shi Y., Pellegrini M., Gaspari M., Wibom R., Hultenby K., Erdjument-Bromage H., Tempst P., et al. MTERF3 is a negative regulator of mammalian mtDNA transcription. Cell. 2007;130:273–285. doi: 10.1016/j.cell.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 25.Spåhr H., Habermann B., Gustafsson C.M., Larsson N.G., Hallberg B.M. Structure of the human MTERF4-NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc. Natl. Acad. Sci. USA. 2012;109:15253–15258. doi: 10.1073/pnas.1210688109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babiychuk E., Vandepoele K., Wissing J., Garcia-Diaz M., De Rycke R., Akbari H., Joubès J., Beeckman T., Jänsch L., Frentzen M., et al. Plastid gene expression and plant development require a plastidic protein of the mitochondrial transcription termination factor family. Proc. Natl. Acad. Sci. USA. 2011;108:6674–6679. doi: 10.1073/pnas.1103442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleine T. Arabidopsis thaliana mTERF proteins: Evolution and functional classification. Front. Plant Sci. 2012;3:233. doi: 10.3389/fpls.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robles P., Micol J.L., Quesada V. Arabidopsis MDA1, a nuclear-encoded protein, functions in chloroplast development and abiotic stress responses. PLoS ONE. 2012;7:e42924. doi: 10.1371/journal.pone.0042924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y., Cai M., Zhang X., Li Y., Zhang J., Zhao H., Kong F., Zheng Y., Qiu F. Genome-Wide identification, evolution and expression analysis of mTERF gene family in maize. PLoS ONE. 2014;9:e94126. doi: 10.1371/journal.pone.0094126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang B., Xie L., Yi T., Lv J., Yang H., Cheng X., Liu F., Zou X. Genome-Wide Identification and Characterization of the Mitochondrial Transcription Termination Factors (mTERFs) in Capsicum annuum L. Int. J. Mol. Sci. 2020;21:269. doi: 10.3390/ijms21010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleine T., Leister D. Emerging functions of mammalian and plant mTERFs. Biochim. Biophys. Acta. 2015;1847:786–797. doi: 10.1016/j.bbabio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Quesada V., Sarmiento-Mañús R., González-Bayón R., Hricová A., Pérez-Marcos R., Graciá-Martínez E., Medina-Ruiz L., Leyva-Díaz E., Ponce M.R., Micol J.L. Arabidopsis RUGOSA2 encodes an mTERF family member required for mitochondrion, chloroplast and leaf development. Plant J. 2011;68:738–753. doi: 10.1111/j.1365-313X.2011.04726.x. [DOI] [PubMed] [Google Scholar]
- 33.Romani I., Manavski N., Morosetti A., Tadini L., Maier S., Kühn K., Ruwe H., Schmitz-Linneweber C., Wanner G., Leister D., et al. A Member of the Arabidopsis Mitochondrial Transcription Termination Factor Family Is Required for Maturation of Chloroplast Transfer RNAIle(GAU) Plant Physiol. 2015;169:627–646. doi: 10.1104/pp.15.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robles P., Núñez-Delegido E., Ferrández-Ayela A., Sarmiento-Mañús R., Micol J.L., Quesada V. Arabidopsis mTERF6 is required for leaf patterning. Plant Sci. 2018;266:117–129. doi: 10.1016/j.plantsci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Robles P., Micol J.L., Quesada V. Mutations in the plant-conserved MTERF9 alter chloroplast gene expression, development and tolerance to abiotic stress in Arabidopsis thaliana. Physiol. Plant. 2015;154:297–313. doi: 10.1111/ppl.12307. [DOI] [PubMed] [Google Scholar]
- 36.Kim M., Lee U., Small I., des Francs-Small C.C., Vierling E. Mutations in an Arabidopsis mitochondrial transcription termination factor-related protein enhance thermotolerance in the absence of the major molecular chaperone HSP101. Plant Cell. 2012;24:3349–3365. doi: 10.1105/tpc.112.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shevtsov S., Nevo-Dinur K., Faigon L., Sultan L.D., Zmudjak M., Markovits M., Ostersetzer-Biran O. Control of organelle gene expression by the mitochondrial transcription termination factor mTERF22 in Arabidopsis thaliana plants. PLoS ONE. 2018;13:e0201631. doi: 10.1371/journal.pone.0201631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan Z., Ren X., Zhao H., Liu L., Tan Z., Qiu F. A Mitochondrial Transcription Termination Factor, ZmSmk3, is Required for nad1 Intron4 and nad4 Intron1 Splicing and Kernel Development in Maize. G3 (Bethesda) 2019;9:2677–2686. doi: 10.1534/g3.119.400265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meskauskiene R., Würsch M., Laloi C., Vidi S., Coll N., Kessler F., Baruah A., Kim C., Apel K. A mutation in the Arabidopsis mTERF-related plastid protein SOLDAT10 activates retrograde signaling and suppresses 1O2-induced cell death. Plant J. 2009;60:399–410. doi: 10.1111/j.1365-313X.2009.03965.x. [DOI] [PubMed] [Google Scholar]
- 40.Quesada V. The roles of mitochondrial transcription termination factors (MTERFs) in plants. Physiol. Plant. 2016;157:389–399. doi: 10.1111/ppl.12416. [DOI] [PubMed] [Google Scholar]
- 41.Robles P., Navarro-Cartagena S., Ferrández-Ayela A., Núñez-Delegido E., Quesada V. The characterization of Arabidopsis mterf6 mutants reveals a new role for mTERF6 in tolerance to abiotic stress. Int. J. Mol. Sci. 2018;19:2388. doi: 10.3390/ijms19082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu D., Leister D., Kleine T. Arabidopsis thaliana mTERF10 and mTERF11, but not mTERF12, are involved in the response to salt stress. Front. Plant Sci. 2017;8:1213. doi: 10.3389/fpls.2017.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wobbe L. The Molecular Function of Plant mTERFs as Key Regulators of Organellar Gene Expression. Plant Cell Physiol. 2020 doi: 10.1093/pcp/pcaa132. [DOI] [PubMed] [Google Scholar]
- 45.Ding S., Zhang Y., Hu Z., Huang X., Zhang B., Lu Q., Wen X., Wang Y., Lu C. mTERF5 Acts as a Transcriptional Pausing Factor to Positively Regulate Transcription of Chloroplast psbEFLJ. Mol. Plant. 2019;12:1259–1277. doi: 10.1016/j.molp.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Cui Y.L., Zhang X.L., Yu Q.B., Wang X., Yuan X.B., Qin X.M., He X.F., Huang C., Yang Z.N. A nuclear-encoded protein, mTERF6, mediates transcription termination of rpoA polycistron for plastid-encoded RNA polymerase-dependent chloroplast gene expression and chloroplast development. Sci. Rep. 2018;8:11929. doi: 10.1038/s41598-018-30166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong H.B., Wang J., Huang C., Rochaix J.D., Lin F.M., Zhang J.X., Ye L.S., Shi X.E., Yu Q.B., Yang Z.N. mTERF8, a Member of the Mitochondrial Transcription Termination Factor Family, Is Involved in the Transcription Termination of Chloroplast Gene psbJ. Plant Physiol. 2020;182:408–423. doi: 10.1104/pp.19.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu Y.W., Wang H.J., Hsieh M.H., Hsieh H.L., Jauh G.Y. Arabidopsis mTERF15 is required for mitochondrial nad2 intron 3 splicing and functional complex I activity. PLoS ONE. 2014;9:e112360. doi: 10.1371/journal.pone.0112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schonfeld C., Wobbe L., Borgstadt R., Kienast A., Nixon P.J., Kruse O. The nucleus-encoded protein MOC1 is essential for mitochondrial light acclimation in Chlamydomonas reinhardtii. J. Biol. Chem. 2004;279:50366–50374. doi: 10.1074/jbc.M408477200. [DOI] [PubMed] [Google Scholar]
- 50.Wobbe L., Nixon P.J. The mTERF protein MOC1 terminates mitochondrial DNA transcription in the unicellular green alga Chlamydomonas reinhardtii. Nucleic Acids Res. 2013;41:6553–6567. doi: 10.1093/nar/gkt313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Méteignier L.V., Ghandour R., Zimmerman A., Kuhn L., Meurer J., Zoschke R., Hammani K. Arabidopsis mTERF9 protein promotes chloroplast ribosomal assembly and translation by establishing ribonucleoprotein interactions in vivo. Nucleic Acids Res. 2021 doi: 10.1093/nar/gkaa1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leister D., Kleine T. Extending the Repertoire of mTERF Proteins with Functions in Organellar Gene Expression. Mol. Plant. 2020;13:817–819. doi: 10.1016/j.molp.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Greger I.H., Aranda A., Proudfoot N. Balancing transcriptional interference and initiation on the GAL7 promoter of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2000;97:8415–8420. doi: 10.1073/pnas.140217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richard P., Manley J.L. Transcription termination by nuclear RNA polymerases. Genes Dev. 2009;23:1247–1269. doi: 10.1101/gad.1792809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chi W., He B., Manavski N., Mao J., Ji D., Lu C., Rochaix J.D., Meurer J., Zhang L. RHON1 mediates a Rho-like activity for transcription termination in plastids of Arabidopsis thaliana. Plant Cell. 2014;26:4918–4932. doi: 10.1105/tpc.114.132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adelman K., Lis J.T. Promoter-proximal pausing of RNA polymerase II: Emerging roles in metazoans. Nat. Rev. Genet. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meurer J., Berger A., Westhoff P. A nuclear mutant of Arabidopsis with impaired stability on distinct transcripts of the plastid psbB, psbD/C, ndhH, and ndhC operons. Plant Cell. 1996;8:1193–1207. doi: 10.1105/tpc.8.7.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Longevialle A.F., Small I.D., Lurin C. Nuclearly encoded splicing factors implicated in RNA splicing in higher plant organelles. Mol. Plant. 2010;3:691–705. doi: 10.1093/mp/ssq025. [DOI] [PubMed] [Google Scholar]
- 59.Williams P.M., Barkan A. A chloroplast-localized PPR protein required for plastid ribosome accumulation. Plant J. 2003;36:675–686. doi: 10.1046/j.1365-313X.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 60.Watkins K., Kroeger T., Cooke A., Williams-Carrier R., Friso G., Belcher S., van Wijk K., Barkan A. A ribonuclease III domain protein functions in group II intron splicing in maize chloroplasts. Plant Cell. 2007;19:2606–2623. doi: 10.1105/tpc.107.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroeger T., Watkins K., Friso G., van Wijk K., Barkan A. A plant-specific RNA binding domain revealed through analysis of chloroplast group II intron splicing. Proc. Natl. Acad. Sci. USA. 2009;106:4537–4542. doi: 10.1073/pnas.0812503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beick S., Schmitz-Linneweber C., Williams-Carrier R., Jensen B., Barkan A. The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol. Cell. Biol. 2008;28:5337–5347. doi: 10.1128/MCB.00563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barkan A. Nuclear mutants of maize with defects in chloroplast polysome assembly have altered chloroplast RNA metabolism. Plant Cell. 1993;5:389–402. doi: 10.2307/3869720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walbot V., Coe E.H. Nuclear gene iojap conditions a programmed change to ribosome-less plastids in Zea mays. Proc. Natl. Acad. Sci. USA. 1979;76:2760–2764. doi: 10.1073/pnas.76.6.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenkins B.D., Kulhanek D.J., Barkan A. Nuclear mutations that block group II RNA splicing in maize chloroplasts reveal several intron classes with distinct requirements for splicing factors. Plant Cell. 1997;9:283–296. doi: 10.1105/tpc.9.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown G.G., Colas des Francs-Small C., Ostersetzer-Biran O. Group II intron splicing factors in plant mitochondria. Front. Plant Sci. 2014;5:35. doi: 10.3389/fpls.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Longevialle A.F., Meyer E.H., Andrés C., Taylor N.L., Lurin C., Millar A.H., Small I.D. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell. 2007;19:3256–3265. doi: 10.1105/tpc.107.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alamdari K., Fisher K.E., Sinson A.B., Chory J., Woodson J.D. Roles for the chloroplast-localized pentatricopeptide repeat protein 30 and the ‘mitochondrial’ transcription termination factor 9 in chloroplast quality control. Plant J. 2020;104:735–751. doi: 10.1111/tpj.14963. [DOI] [PubMed] [Google Scholar]
- 69.Mokry M., Nijman I.J., van Dijken A., Benjamins R., Heidstra R., Scheres B., Cuppen E. Identification of factors required for meristem function in Arabidopsis using a novel next generation sequencing fast forward genetics approach. BMC Genom. 2011;12:256. doi: 10.1186/1471-2164-12-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shikanai T. RNA editing in plant organelles: Machinery, physiological function and evolution. Cell. Mol. Life Sci. 2006;63:698–708. doi: 10.1007/s00018-005-5449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shikanai T., Fujii S. Function of PPR proteins in plastid gene expression. RNA Biol. 2013;10:1446–1456. doi: 10.4161/rna.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]