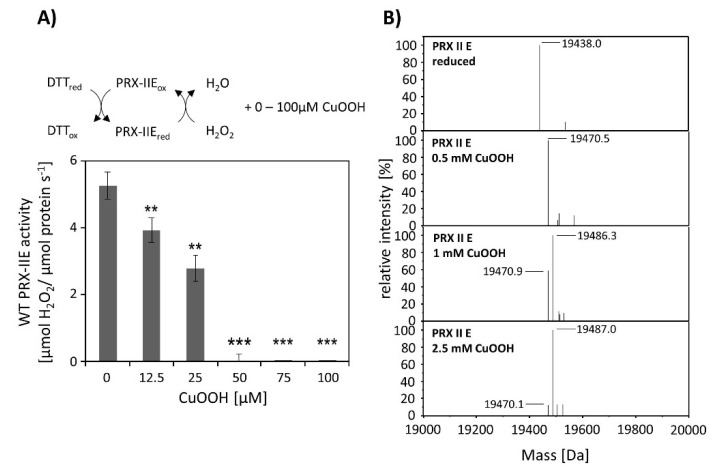
Figure 3.
Hyperoxidation of PRX-IIE. (A) Activity of 2 µM PRX-IIE was determined with 400 µM H2O2 as substrate and 4 mM DTT using the FOX assay. CuOOH concentrations > 50 µM completely inhibited the peroxidase activity. Data are means of n = 9 ± SD with protein of three independent protein purifications. **: p < 0.01; ***: p < 0.001. (B) Deconvoluted ESI-MS spectra of wild-type PRX-IIE after treatment with CuOOH as described in Materials and Methods. 19,438.0 is the expected mass of the reduced (-SH), His-tagged PRX-IIE protein. The peak at 19,470.5 Da after incubation with 0.5 mM CuOOH shows a mass increase by 32 Da, which corresponds to the formation of the sulfinic acid derivative (-SO2H). Higher CuOOH concentrations lead to further oxidation to the sulfonic acid (-SO3H). Data are representative spectra of n = 15 measurements with the protein of three independent protein purifications.