Fig. 1. On-bead BS3 cross-linking reveals a highly interlinked complex.
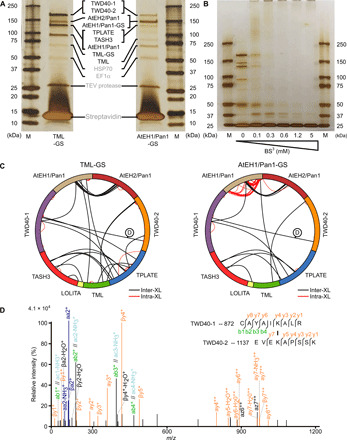
(A) Tandem affinity purification of TPC using TML- and AtEH1/Pan1-tagged subunits. The purified complex was analyzed by silver stain on a 4 to 20% SDS-PAGE. All TPC subunits, except LOLITA, were identified on the basis of MS (data file S8) and are indicated between both gels. M, marker; HSP70, heat shock protein 70. EF1, elongation factor 1; TEV, Tobacco Etch Virus. (B) Tandem affinity–purified TML-GS before and after cross-linking with various concentrations of BS3, analyzed by silver staining on a 4 to 20% SDS-PAGE gel. The vast size of TPC, with an expected molecular weight of 914 kDa, is manifested by the loss of individual subunits and the accumulation of proteins unable to penetrate the stacking gel. (C) Cross-linking analysis following tandem purification of TML- and AtEH1/Pan1-tagged subunits expressed in PSB-D cell cultures visualized by Xvis. Each analysis originates from a total of six experiments and combines 1.2 and 5 mM BS3 datasets. (D) An example of the fragmentation spectrum of the intersubunit cross-link between TWD40-1 and TWD40-2, as indicated in (C).
