Figure 6.
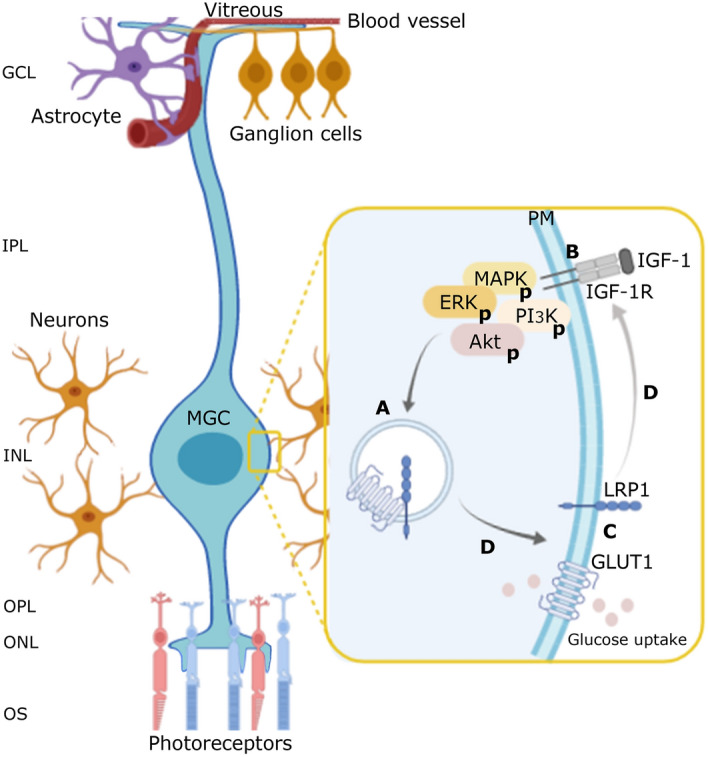
Schematic model of LRP1 mediation in GLUT1 translocation to cell surface and glucose uptake induced by IGF-1. (A) Representative image in which, in non-stimulated MIO-M1 cells, LRP1 and GLUT1 are stored in same, but uncharacterized vesicles, since they are molecularly associated through a possible direct interaction or mediated by adaptor proteins. This molecular association would be necessary to retain GLUT1 inside the cells. (B) IGF-1 induces MAPK/ERK and PI3K/Akt signaling activation through its cognate receptor (IGF-1R). (C) This IGF-1-induced activation promptly leads to the molecular dissociation of LRP1 and GLUT1, promoting the intracellular traffic of both membrane proteins to the PM and glucose uptake. Nevertheless, if both intracellular signaling pathways have different downstream targets on the GLUT1 traffic are still unknown. (D) The LRP1 knockdown fully abrogates the IGF-1R intracellular signaling, the GLUT1 translocation and glucose uptake processes. Taken account these considerations, we propose that the LRP1 mediation in the IGF-1-induced glucose control in MIO-M1 cells may be focused at two levels: (1) by regulating the intracellular traffic of GLUT1, and (2) by acting as a scaffold protein for the IGF-1R activation. Ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), outer segment layer (OS).
