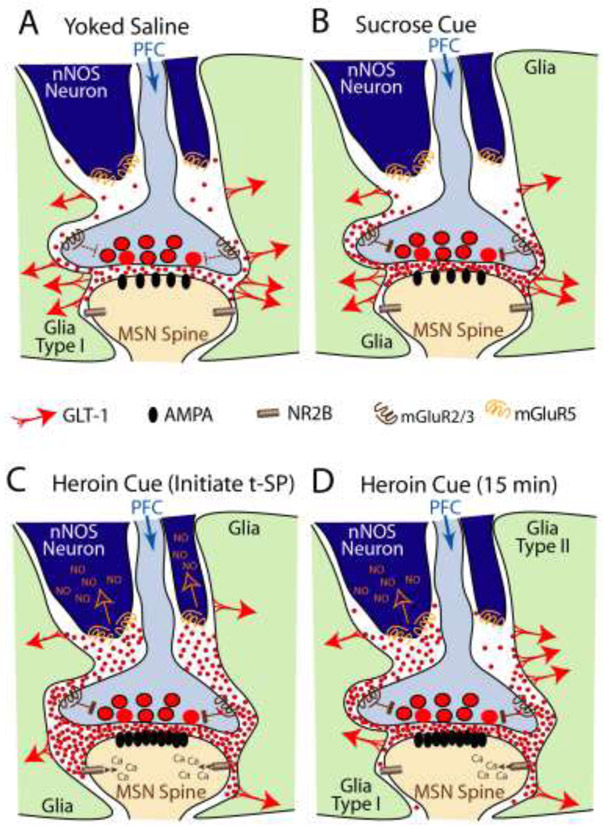
Figure 1. Hypothesized model of how astroglial adaptations impact reinstated heroin seeking.
A) Following yoked saline delivery, synaptic glutamate release is easily accommodated by the presence of GLT-1 on astroglial processes near the synapse. Perisynaptic astroglial insulation favors the postsynapse, biasing glutamate spillover towards presynaptic mGluR2/3 autoreceptors that regulate release. B) Although operant training with sucrose reduces surface expression of GLT-1, synaptic proximity by astrocyte processes is maintained, leading to sufficient glutamate uptake and mGluR2/3 autoreceptor stimulation to prevent synaptic spillover from activating mGluR5 on nNOS interneurons or extrasynaptic NR2B on the postsynapse in response to sucrose-associated cues. C) Following heroin withdrawal there is both reduced GLT-1 and reduced astroglial proximity to the synapse. This allows for maximal spillover and glutamatergic stimulation of mGluR5 on nNOS interneurons and extrasynaptic NR2B, initiating postsynaptic potentiation through AMPA receptor insertion, and amplifying the behavioral motivation to reinstate heroin seeking. D) By 15 min of cued heroin reinstatement, astroglia have undergone two distinct adaptations that each partly dampen reinstated heroin seeking via distinct mechanisms. In type I astroglia, synaptic proximity returns, presumably to the postsynapse that biases access of synaptic glutamate spillover towards mGluR2/3 autoreceptors and away from postsynaptic NR2B. However, we hypothesize that more distant diffusion of glutamate to mGluR5 on nNOS interneurons to amplify reinstated heroin seeking is not prevented. Conversely, in type II astroglia GLT-1 on the surface is increased extrasynaptically, perhaps impairing diffusion of glutamate spillover to extrasynaptic mGluR5 on nNOS interneurons, limiting postsynaptic potentiation.