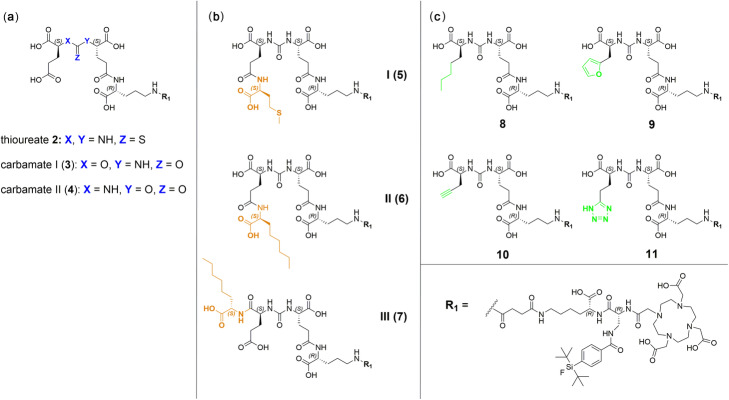
Fig. 2.
Detailed structures of the modified PSMA inhibitors investigated in this study, with (a) thioureate 2, carbamate I & II (3 & 4) (b) proinhibitors I, II and III (5, 6 & 7) and (c) L-2-aminoheptanoic acid (8), furyl (9), alkyne (10) and tetrazole (11) derivatives. All compounds are depicted in their free chelator form and represent PSMA ligands containing modifications within the central Zn2+-binding unit (a), proinhibitor motifs (b) and substituents & bioisosteres of the P1’-γ-carboxylic acid (c)